Efficacy and Safety of Everolimus with Reduced-Dose Calcineurin Inhibitor in De Novo Kidney Transplant Recipients: Results from the TRANSFORM Study
Julio Pascual1, Steve Chadban2, Helio Tedesco3, Stefan Berger4, Yasir Qazi5, Josep M Cruzado6, Peter Bernhardt7, Christophe Legendre8, Myoung Soo Kim9, Flavio Vincenti10.
1Hospital del Mar, Barcelona, Spain; 2Royal Prince Alfred Hospital, Camperdown, Australia; 3Hospital do Rim e da Hipertensão, São Paulo, Brazil; 4University Medical Centre Groningen, Groningen, Netherlands; 5University of Southern California, Los Angeles, CA, United States; 6University Hospital of Bellvitge, Barcelona, Spain; 7Novartis Pharma AG, Basel, Switzerland; 8Hôpital Necker, Paris, France; 9Severance Hospital, Seoul, Korea; 10UC San Francisco Medical Centre, San Francisco, CA, United States
TRANSFORM Study Group.
Introduction: Strategies that facilitate calcineurin inhibitor (CNI) reduction while retaining immunosuppressive efficacy and improving long-term outcomes are highly desired in kidney transplant recipients (KTxRs). TRANSFORM (NCT01950819) is the largest prospective study in de novo KTxRs till date to compare the efficacy and safety of everolimus (EVR)+reduced-dose CNI (rCNI: tacrolimus [TAC] or cyclosporine [CsA]) regimen to that of mycophenolic acid (MPA)+standard-dose CNI (sCNI) using a composite endpoint of anti-rejection efficacy and renal function. Here, we present the 12-month (M) efficacy and safety data from TRANSFORM.
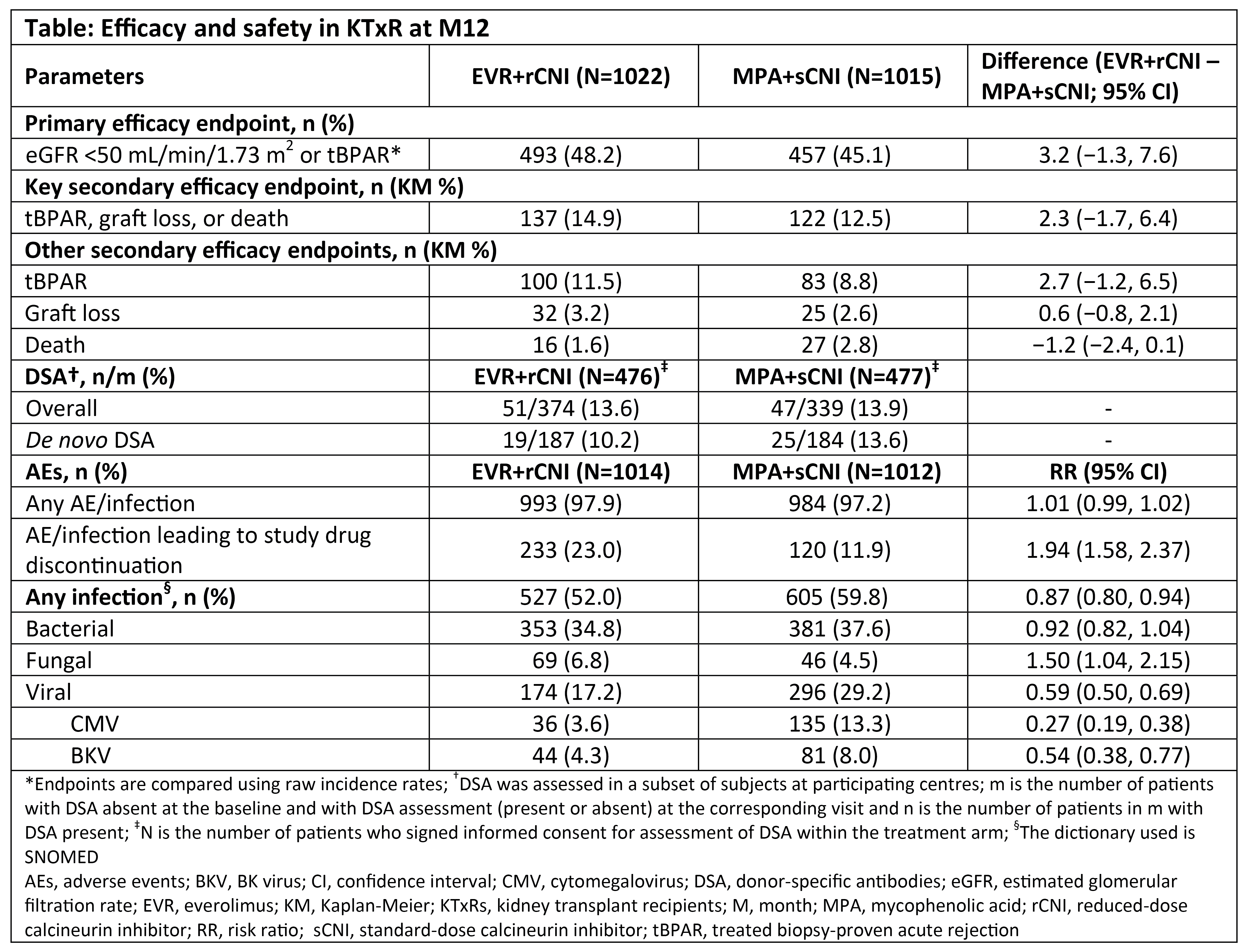
Methods: TRANSFORM is a 24M, multicentre, open-label, non-inferiority study in which 2037 KTxRs were randomised to receive either EVR (dose: 1.5 and 3 mg/day with CsA and TAC, respectively; trough level [C0]: 3-8 ng/mL)+rCNI (N=1022; TAC C0: 4-7, 2-5, and 2-4 ng/mL and CsA C0: 100-150, 50-100, and 25-50 ng/mL from Day1-M2, M3-M6, and M7-M24, respectively) or MPA (dose: 1.44 and 2 g/day for enteric-coated mycophenolate sodium and mycophenolate mofetil, respectively)+sCNI (N=1015; TAC C0: 8-12, 6-10, and 5-8 ng/mL and CsA C0: 200-300, 150-200, and 100-200 ng/mL from Day1-M2, M3-M6, and M7-M24, respectively). All patients received basiliximab or anti-thymocyte globulin induction with steroids. The primary objective was incidence of binary composite of treated biopsy-proven acute rejection (tBPAR) or estimated glomerular filtration rate (eGFR) <50 mL/min/1.73m2; key secondary objective was incidence of tBPAR, graft loss, or death at M12. Incidences of donor-specific antibodies (DSA), adverse events (AEs) and infections were also evaluated.
Results: Overall, 76.9% KTxRs (EVR+rCNI: 72.7%; MPA+sCNI: 81.2%) completed the study medication up to M12. Mean EVR C0 was within target range throughout the study, whereas mean TAC C0 was above the upper limit of the target range in the EVR+rCNI arm and within range in the MPA+sCNI arm. EVR+rCNI was non-inferior (10% margin) to MPA+sCNI (48.2% vs 45.1%, P=0.001) for the primary endpoint (Table). Overall incidence of tBPAR was low, with most episodes of grade IA. Graft loss was comparable between arms, whereas deaths were numerically lower in EVR+rCNI (1.6%) vs MPA+sCNI arm (2.8%; Table). Mean eGFR over M12 was comparable between arms. Incidence of de novo DSA was comparable between arms (EVR+rCNI: 10.2%; MPA+sCNI: 13.6%). Overall AEs were comparable between arms; incidence of AEs leading to study drug discontinuation was higher with EVR+rCNI vs MPA+sCNI, but more dose reduction in MPA+sCNI arm. BK virus and cytomegalovirus (CMV) infections were less frequent in EVR+rCNI vs MPA+sCNI arm.
Conclusion: M12 results from the TRANSFORM study confirm that an EVR+rCNI-based regimen provides comparable efficacy, safety, and renal function vs an MPA+sCNI-based regimen in KTxRs along with benefit against viral infections. M24 follow-up data on long-term efficacy, safety, and renal function are awaited.