Nicole M Shockcor, United States has been granted the TTS Young Investigator Scientific Award

End Stage Renal Disease as a Complication of Face Transplant
Nicole Shockcor1, Bryan Buckingham1, Wessam Hassanein1, Abdolreza Haririan1, Sean M Roberts2, Arthur J Nam1, Eduardo D Rodriquez3, Stephen T Bartlett1, Rolf N Barth1.
1Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, United States; 2Department of Medicine, Ochsner Health Center, Covington, LA, United States; 3Department of Plastic Surgery, New York University Langone Medical Center, New York, NY, United States
Introduction: Renal failure as an outcome of chronic immunosuppression is a known complication of organ transplantation. We report the first clinical case of end stage renal disease resulting from calcineurin inhibitor (CNI) based immunosuppression in vascularized composite allotransplantation (VCA).
Materials and Methods: A full-face transplant was performed with maxilla, mandible, tongue, skin, and scalp on March 2012. Induction immunosuppression was provided by alemtuzumab and maintenance therapy with tacrolimus, mycophenolate mofetil, and prednisone. Tacrolimus target levels of 6-8 ng/mL during the first year were lowered to 5 ng/mL in subsequent years. The patient maintained regular nephrology and transplant follow up.
Results and Discussion: Three clinically significant graft rejection episodes occurred on postoperative days 28, 402, and 712 with BANFF I, I, and II scores respectively. Episode 1 occurred after weaning steroids, episode 2 after sub-therapeutic tacrolimus levels, and the most severe episode 3 after decreased tacrolimus and introduction of everolimus as a renal protective strategy. After year 2 no further rejection episodes occurred with maintenance therapy MMF 500 mg bid, prednisone 5 mg daily, and a tacrolimus target level of 5 ng/mL. Creatinine preoperatively was noted to be 0.6-1.1 mg/dL. Year 2 average creatinine levels remained elevated at 2 mg/dL. Year 5 creatinine increased to 5 mg/dL with non-oliguric CKD Stage 5 and an eGFR of 15 mL/min/BSA. 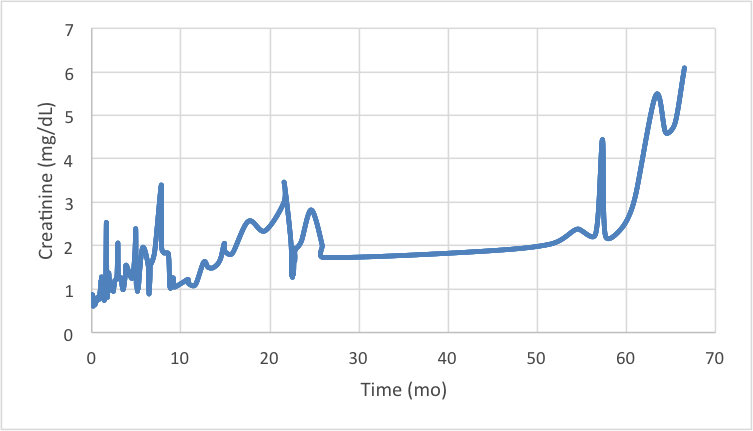 CT imaging revealed diminishing size of native kidneys consistent with renal disease. The patient has been listed for renal transplantation. Biopsy at year 5 demonstrated no evidence of rejection or chronic vasculopathy. Clinical appearance and function remained excellent.
CT imaging revealed diminishing size of native kidneys consistent with renal disease. The patient has been listed for renal transplantation. Biopsy at year 5 demonstrated no evidence of rejection or chronic vasculopathy. Clinical appearance and function remained excellent.
Conclusion: This is the first reported clinical case of ESRD as a direct consequence of immunosuppression in VCA. Understanding complications of VCA with standard immunosuppression is critical in risk/benefit considerations for VCA candidates. Continued research into alternate immunosuppressive approaches to achieve excellent graft outcomes with minimal complications is critical to the future success of this field.
Astellas Pharma US, Inc.. Henry Jackson Foundation, Department of Defense Award # W81XWH-13-2-0056.
