Role of Graft-derived Graft-versus-Host T cells in Facilitating Multilineage Blood Chimerism after Human Intestinal Transplantation
Jianing Fu PhD1,2, Julien Zuber MD, PhD1,3, Brittany Shonts BA1,2, Aleksandar Obradovic BA1, Sai-ping Lau BSc1, Elizabeth Waffarn DVM, PhD1,2, Thomas Savage BA1, Suxiao Yang MS1,2, Kortney Rogers BA1,2, Siu-hong Ho PhD1,2, Yufeng Shen PhD4, Prakash Satwani MD5, Mercedes Martinez MD5, Tomoaki Kato MD6, Megan Sykes MD1,2,6,7.
1Columbia Center for Translational Immunology, Columbia University, New York, NY, United States; 2Department of Medicine, Columbia University, New York, NY, United States; 3Department of Kidney Transplantation, Necker Hospital, Paris, France; 4Center for Computational Biology and Bioinformatics, Department of Systems Biology, Columbia University, New York, NY, United States; 5Department of Pediatrics, Columbia University, New York, NY, United States; 6Department of Surgery, Columbia University, New York, NY, United States; 7Department of Microbiology & Immunology, Columbia University, New York, NY, United States
Long-term multilineage chimerism often appears in blood following human intestinal transplantation (ITx), especially in multivisceral transplant (MVTx) recipients. Patients with donor T cell macrochimerism (>4%) in blood, frequently without graft-versus-host disease (GVHD), displayed less graft rejection and donor-specific antibody (DSA) production and slower graft T cell replacement by the recipient.
To address the hypothesis that the balance of graft-versus-host (GvH) and host-versus-graft (HvG) alloreactivity might underly these relationships, we analyzed the phenotype, repertoire and alloreactivity of donor- and recipient-derived T cells in the intestinal allograft, peripheral blood and bone marrow (BM) of ITx recipients. We identified GvH and HvG clones on the basis of high throughput TCRβ CDR3 sequencing from pre-Tx donor and recipient mixed lymphocyte reactions as we have described1.
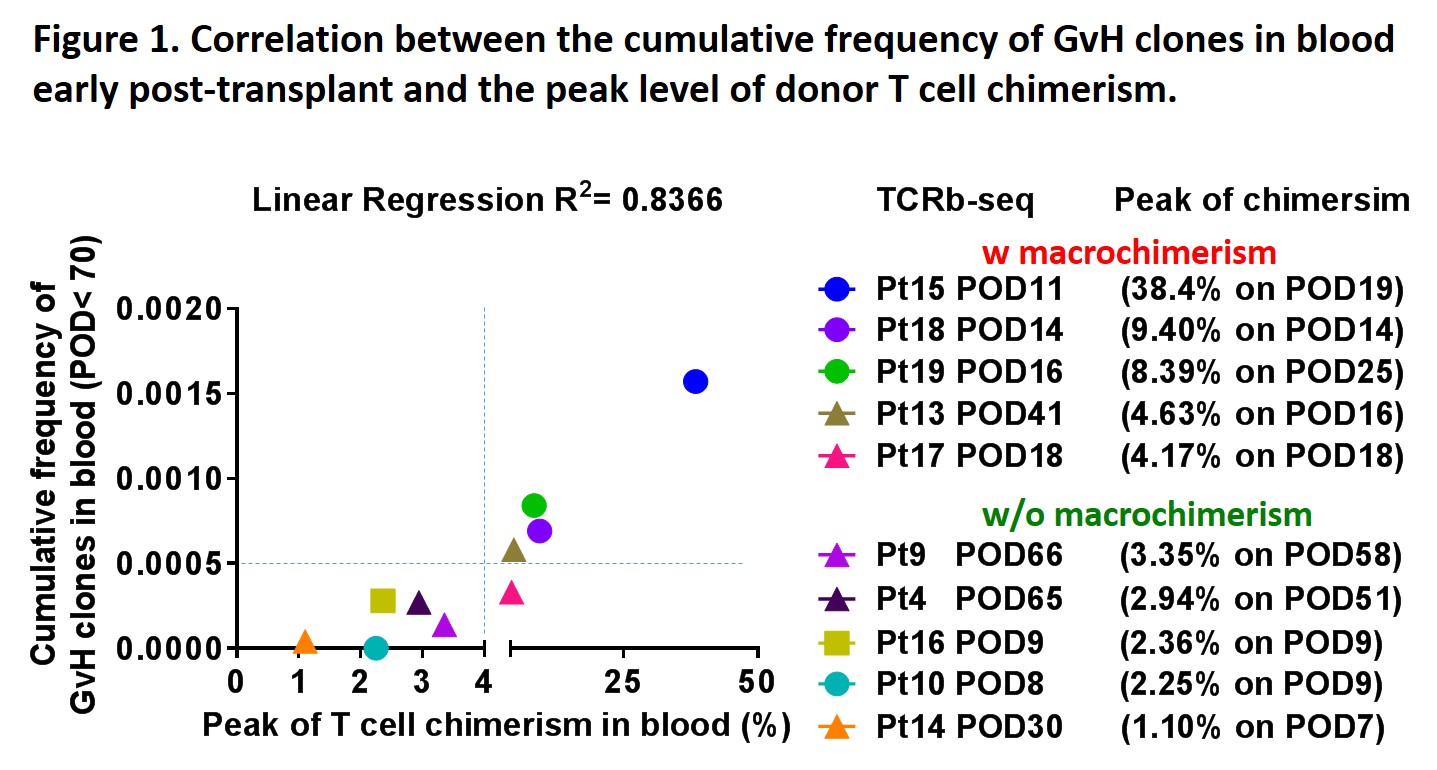
Enrichment of GvH compared to HvG clones in early intestinal biopsies and absence of Class I DSA were associated with donor T cell macrochimerism. Local expansion of GvH clones in the graft was likely driven by uniformly rapid replacement of recipient antigen presenting cells (APCs) within allografts early post-Tx. Cumulative frequency of GvH clones in blood early post-Tx was positively correlated with the peak level of donor T cell chimerism (Fig.1).
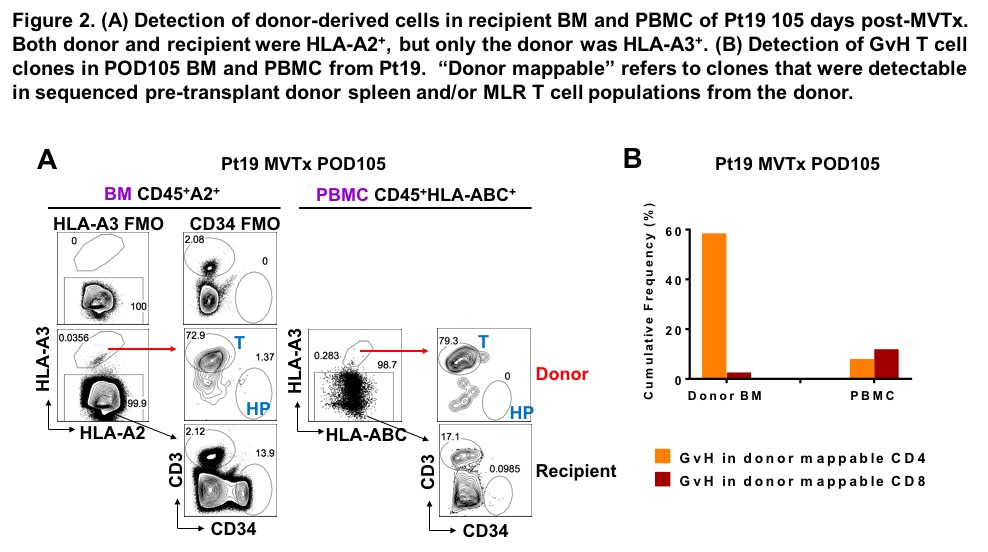
Unlike donor ileal lymphocytes, which display a tissue-resident memory phenotype, long-term circulating donor T cells were markedly enriched for the recent thymic emigrant phenotype compared with recipient cells, and GvH clones were undetectable among these late circulating naïve donor-derived T cells. Enrichment of donor circulating naïve B cells and T cells and recurrent myeloid chimerism late post-Tx are consistent with our observation that donor-derived hematopoietic stem and progenitor cells are present in intestinal allografts and are detectable in the circulation of patients with macrochimerism. Multilineage chimerism might reflect a lymphohematopoietic GvH response (LGVHR) migrating from recipient circulation to the BM, making “space” for engraftment of progenitor cells from the graft. Indeed, 0.0356% of CD45+ cells in the BM of an MVTx patient on day 105 post-Tx were donor-derived, among which 72.9% were T cells and 1.37% were CD34+ progenitors. TCR sequencing of sorted donor cells in recipient BM identified donor-mappable clones, among CD4s of which 60% by frequency were GvH-reactive. GvH CD4 cells did not show marked enrichment in donor-mappable PBMCs on the same day. (Fig.2)
Together, these data suggest that donor GvH-reactive T cells expanding within the graft in response to recipient APCs entering the graft migrate into the recipient circulation and BM, playing a key role in promoting and maintaining mixed chimerism. LGVHR without GVHD in ITx recipients suggests a novel approach to achieving intestinal allograft tolerance.
1. Zuber et al., 2016 Science Immunology.
The study was funded by the National Institute of Allergy and Infectious Diseases grant P01 AI106697. Research reported in this study was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under awards S10RR027050 and S10OD020056. .
