In Vivo Perfusion of Porcine Kidney Scaffolds Re-Endothelialized with Human Umbilical Vein Endothelial Cells
Matthew L Holzner1, Dylan Adamson1, Brandy Haydel1, Senthuran Atputhanathan2, Pablo D Cabral2, Dominique Seetapun2, Jeff J Ross2, Vikram Wadhera1, Ron Shapiro1, Sander Florman1.
1Recanati/Miller Transplantation Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States; 2Miromatrix Medical Inc., Eden Prairie, MN, United States
Introduction: Tissue engineering is a promising strategy to address the current organ shortage. Whole organ bioengineering utilizes a decellularization process to create an acellular extracellular matrix (ECM) that serves as a scaffold upon which cells can be repopulated. Recellularizing acellular scaffolds with functional endothelium is a barrier to bioengineering transplantable kidneys. We report on the in vivo perfusion viability of porcine kidney scaffolds re-endothelialized with human umbilical vein endothelial cells (HUVECs) in a pre-clinical large animal model.
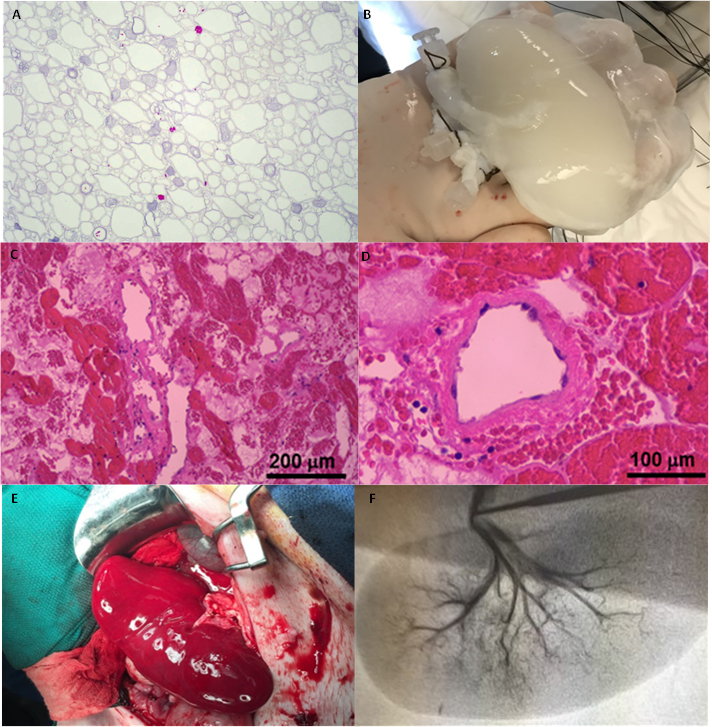
Methods: Kidneys harvested from 35kg Yorkshire pigs were cannulated and underwent perfusion decellularization using sodium dodecyl sulfate (SDS) in combination with Triton X-100. This resulted in isolated ECM scaffolds with intact native tubular and vascular architecture. Scaffolds were re-endothelialized with HUVECs via the renal artery and vein under continuous physiological perfusion pressures and cultured in a bioreactor system for 14-51 days. Endothelial coverage was confirmed by CD31 positivity on immunohistochemistry. Re-endothelialized grafts were implanted in recipient pigs in a heterotopic fashion for up to 2 hours prior to explantation. Non-endothelialized scaffolds were also implanted for comparison. Explanted grafts underwent histological analysis and angiography.
Results: Two re-endothelialized grafts were successfully implanted without acute thrombosis. Continuous arterial and venous perfusion was confirmed with intraoperative Doppler ultrasound for 2 hours. Upon explantation, angiography demonstrated the preserved vascular architecture of the scaffolds. H&E staining of explanted grafts showed a diffusely intact endothelial lining with patent vascular lumens. In comparison, non-endothelialized scaffolds thrombosed within 10 minutes after reperfusion with absent vascular flow on Doppler ultrasound.
Conclusion: This is a proof of concept study demonstrating functional in vivo perfusion of re-endothelialized porcine kidney scaffolds with HUEVCs in a large animal model. Further investigation is underway to achieve sustained vascular patency as the next step towards reseeding scaffolds with functional parenchyma.