Human Urine-Derived Stem Cells could Protect Renal Function by Releasing miR-129-5p which Targets High-Mobility Group box-1 (HMGB1) in Rat Ischemia Reperfusion Injury Model
Longshan Liu1, Xirui Li1, Qun Su1, Zirong Bi1, Huiting Huang1, Changxi Wang1.
1Organ Transplant Center, The First Affiliated Hospital of Sun Yat-sen University , Guangzhou, P.R. China
Background: Severe ischemia reperfusion injury (IRI) causes delayed graft function and impaires renal allograft survival. Stem cell therapy shows promising protective effect on renal IRI. Human urine-derived stem cells (USCs) is a new source of stem cells detached from kidneys and may possess tissue-specific previldge in treatment of kidney injury. This study investigated the protective effect of human USCs on renal IRI in rats and explored the underlying mechanism involving the pro-inflammatory cytokine, high-mobility group box-1 (HMGB1).
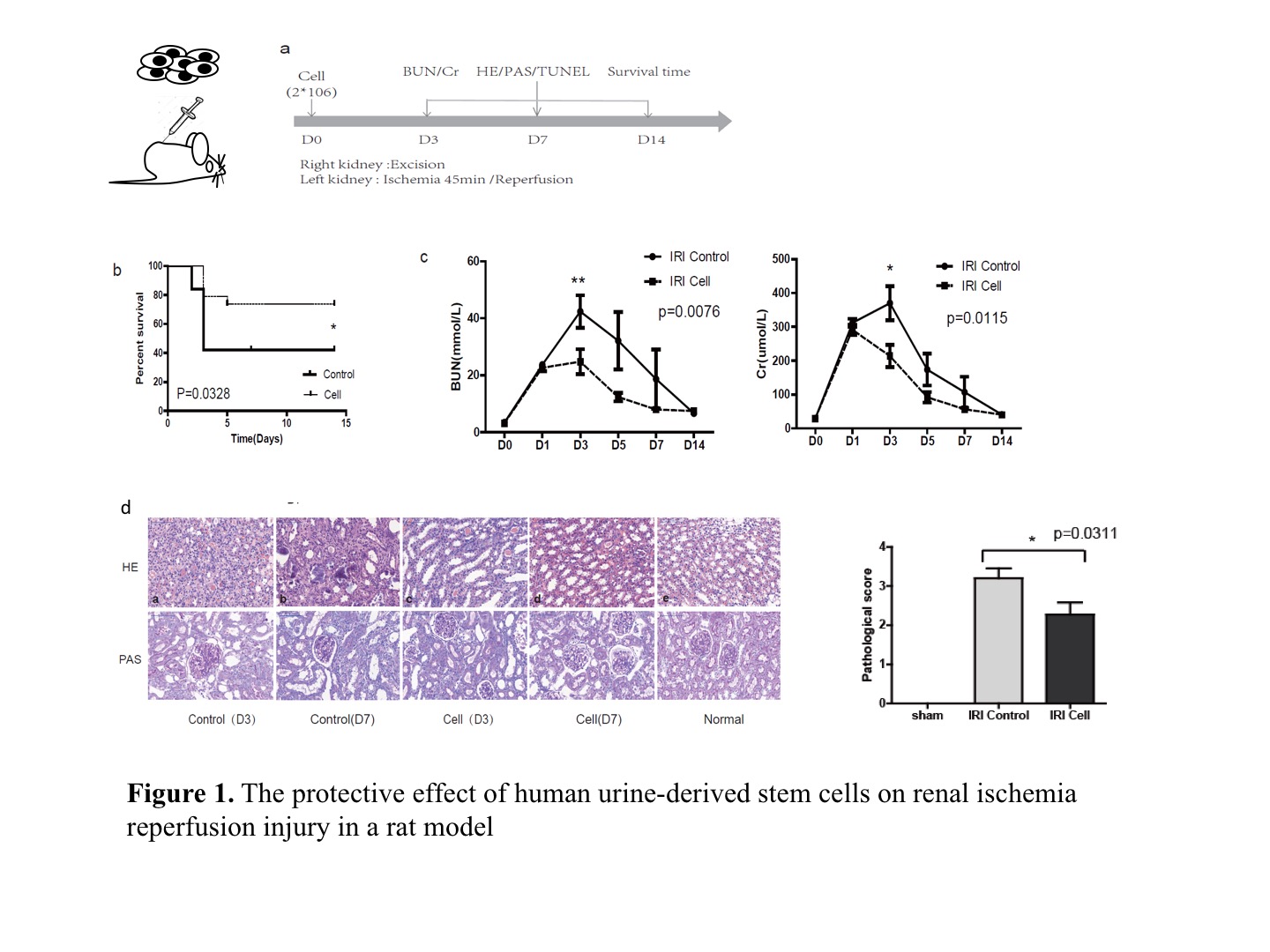
Materials and Methods: Human USCs was cultured and harvested from fresh spot urine. The phenotype of cell surface markers and differenatiation ability was analyzed to determine the characteristics of stem cells. Adult male Sprague-Dawley rats were used to induce a lethal renal IRI model. Left renal pedicles was occluded with a vascular clamp for 45 minutes. The contralateral kidney was nephrectomized after reperfusion of the left kidney. One dose of human USCs (2*106 cells) in the experiment group or saline in the control group was intravenously administered right after blood reperfusion (Fig. 1a).
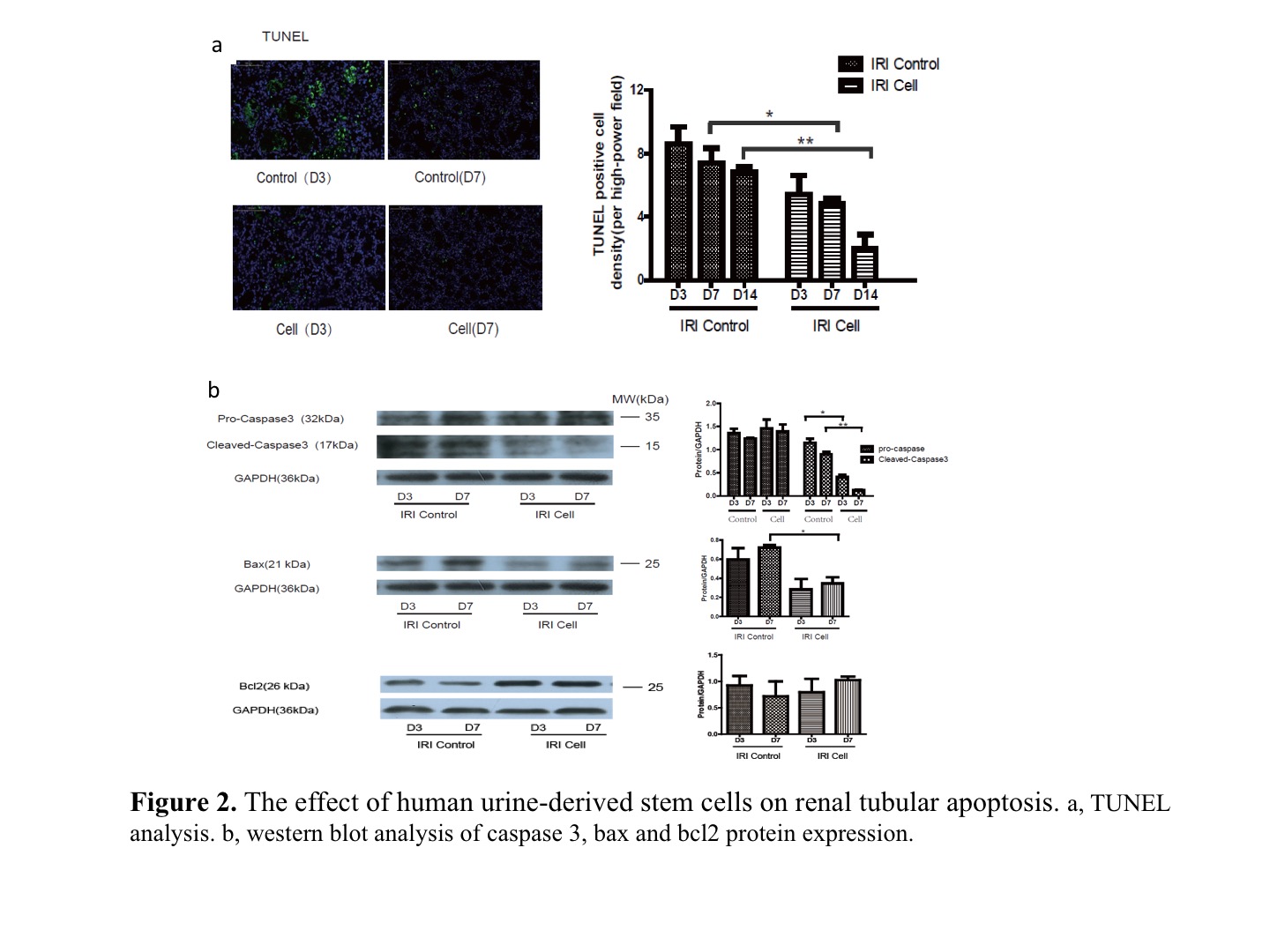
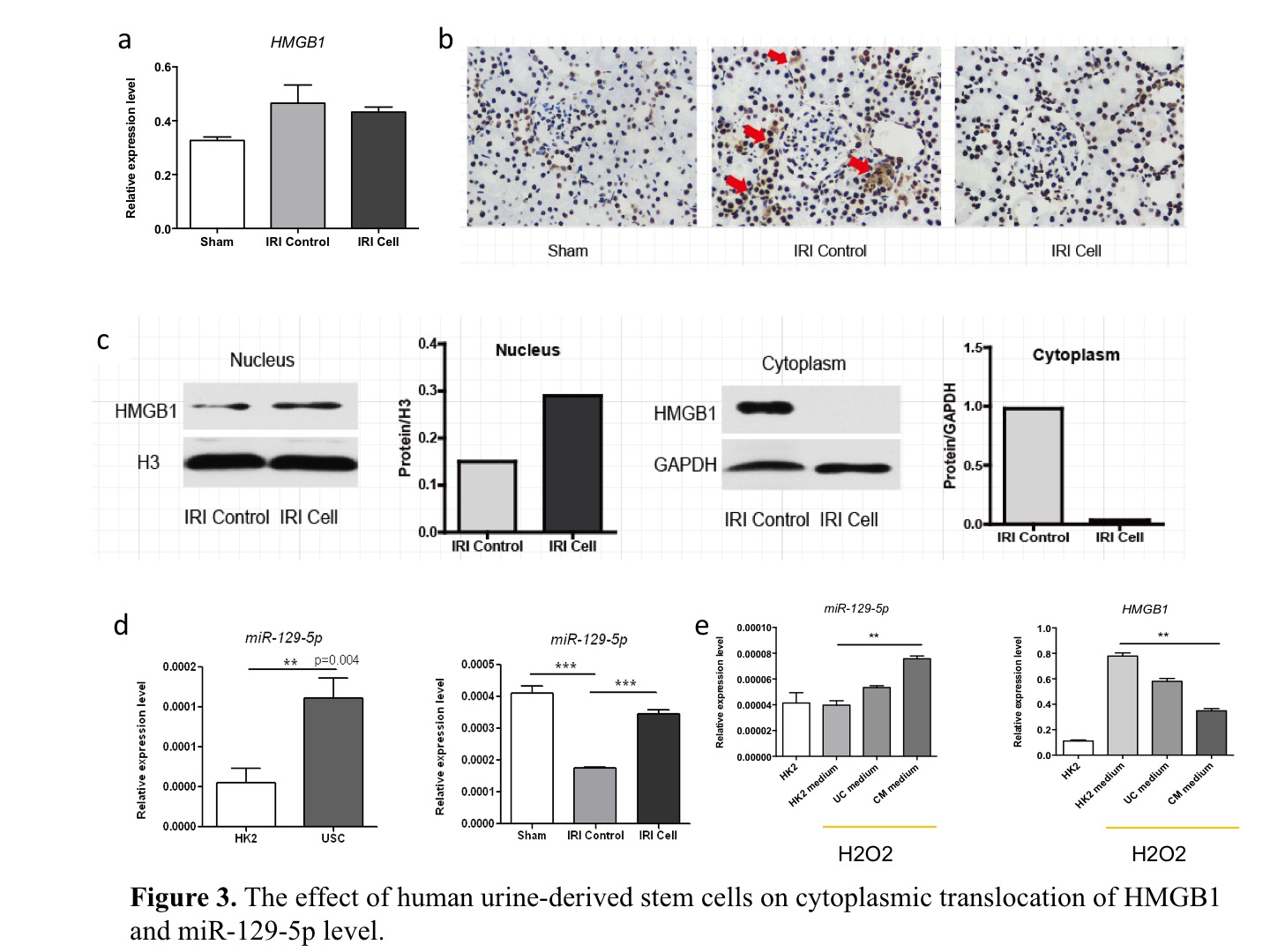
Results: Compared to the control, human USCs significantly increased rat survival rate (Fig. 1b, p=0.03),and decreased serum creatinine and blood urine nitrogen (BUN) from day 3 to day 7 after reperfuion (Fig. 1c, p<0.05). Acute tubular injury score was significantly decreased in USCs group (Fig. 1d, p=0.03). TUNEL analysis showed fewer apoptotic cells in USCs group at day 3 and day 7 (Fig. 2a, p<0.05). The cleaved-caspase 3 and apoptosis-related protein bax were decreased while anti-apoptotic protein bcl2 was increased in USCs group (Fig. 2b, p<0.05). Myeloperoxidase (MPO) staining score was significantly lower in USCs group (p<0.05). Notably, the releasing of HMGB1 from nucleus was significantly decreased in USCs group (Fig. 3b,3c), while its mRNA expression level in total renal tissue was similar to the control (Fig. 3a). Meanwhile, miR-129-5p expression in renal tissue was significantly unregulated in USCs group (Fig. 3d). Furthermore, in an oxidative-stressed HK2 (Human kidney cortex/proximal tubule cells ) cell culture model, USCs conditioned medium significantly increasing miR-129-5p level while reduced HMGB1 (Fig. 3e).
Discussion and Conclusion: Our data demonstrated that human USCs attenuate renal ischemia reperfusion injury in rats. HMGB1 is a classical damage associated molecular patterns(DAMPs)molecular. HMGB1 becomes an active pro-inflammatory factor once it is released extracellularly. Through bioinformatics analysis, we found miR-129-5p could target HMGB1 and reduce HMGB1 level. Thus, we hypothesize human USCs inhibits the expression and releasing of HMGB1 in injury renal tubular cells probably through the release of miR-129-5p and subsequently inhibit infiltration of inflammatory cells.
This work was supported by National Natural Science Foundation of China (81670680), Science and Technology Planning Project of Guangdong Province, China (2014B020212006, 2015B020226002, 2013B051000020, 2013B021800292, 2013B022000055, 2010B031600236), Science and Technology Program of Guangzhou, China (2014Y2-00114) and the Guangdong Provincial Key Laboratory on Organ Donation and Transplant Immunology (2013A 061401007). .
