Clinical Outcomes of Sofosbuvir-Based Direct-Acting Antiviral Therapy in Kidney Transplant Recipients Infected with Hepatitis C Virus
Mohamad Alkadi1, Essa Abuhelaiqa1, Omar Fituri1, Moataz Derbala2, Mona Jarman3, Jehan Mahmoud3, Muhammad Asim1, Adel Aziz1, Awais Nauman1, Ahmed Hamdi1, Saad Al Kaabi2, Hassan Al-Malki1.
1Medicine-Nephrology, Hamad Medical Corporation, Doha, Qatar; 2Medicine-Gastroenterology, Hamad Medical Corporation, Doha, Qatar; 3Surgery-Transplant, Hamad Medical Corporation, Doha, Qatar
Introduction: Hepatitis C virus (HCV) infection has an adverse effect on patient and graft survival in kidney transplant recipients. In the past, treatment of HCV infection was not effective and at the same time carried high risk of acute rejection. However, new direct-acting antiviral (DAA) therapy has transformed treatment of HCV infection and resulted in high response rate. The aim of this study was to retrospectively examine the clinical outcomes of HCV-infected kidney transplant recipients at our center who were treated with DAA therapy.
Methods: We identified fifteen kidney transplant recipients who had positive HCV PCR post transplantation and got treated with DAA therapy. Two patients were co-infected with hepatitis B virus and received entecavir in addition to DAA.
Results: The mean age of treated patients was 48 years (± 12.6 ). 10 patients (67%) were males. 3 of the 15 patients also had liver transplant. 67% of patients acquired HCV infection post transplantation. 7 patients (47%) had HCV genotype 1, 5 patients (33%) genotype 4 and 3 patients (20%) genotype 3. None of the patients were positive for HIV infection. All patients had estimated GFR more than 30 ml/min/1.73 m2. Patients were treated with sofosbuvir-based regimen: 8 patients (53%) with ledipasvir/sofosbuvir, 6 patients (40%) with daclatasvir/sofosbuvir and 1 patient with simeprevir/sofosbuvir. The duration of DAA treatment was 3-6 months. All patients had undetected HCV at 8 weeks of treatment and 12-week sustained virologic response (SVR). The treatment was well tolerated and there was no significan graft dysfunction in any of the treated patients as shown in the figure below.
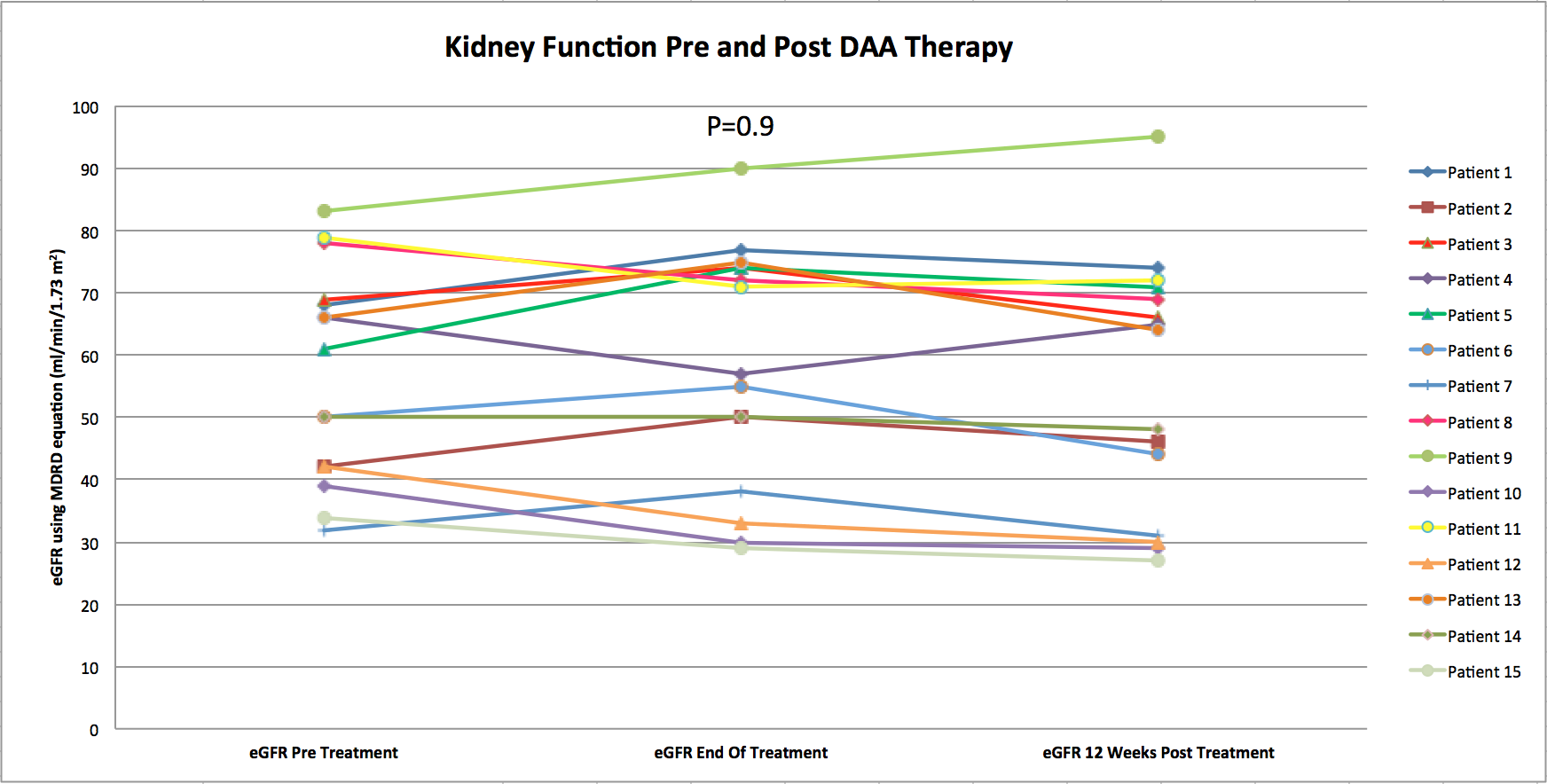
Conclusion: 100% of HCV-infected kidney transplant recipients at our center achieved 12-week SVR with Sofosbuvir-based DAA therapy with no major side effects or significant graft dysfunction.
