Challenges in Calcineurin Inhibitor (CNI) Withdrawal in Conjunction with Co-Stimulation Blockade in Simultaneous Pancreas and Kidney Transplant (SPK)
P G Stock MD, PhD1, R B Mannon MD2, B Armstrong PhD3, N Watson MS4, D Ikle PhD3, M Robian MD4, Y Morrison MS4, J Odorico MD5, J Fridell MD6, Casey Ward1, Q Tang PhD1, A K Mehta MD7, K A Newell MD, PhD7.
1UCSF, San Francisco, CA, United States; 2UAB, Birmingham, AL, United States; 3Rho Inc., Chapel Hill, NC, United States; 4NIH/NIAID, Bethesda, MD, United States; 5UW, Madison, WI, United States; 6IU, Indianapolis, IN, United States; 7Emory University, Atlanta, GA, United States
NIH CTOT 15.
Introduction: Simultaneous pancreas-kidney transplants (SPK) are dependent on calcineurin inhibitors (CNI) to block the alloimmune and recurrent autoimmune response following transplant. Ironically, CNIs are diabetogenic and toxic to the kidney, negatively impacting long-term allograft function. The NIH multicenter CTOT-15 study tested the hypothesis that thymoglobulin induction and co-stimulation blockade with belatacept (bela) could minimize and eliminate CNI and steroids following SPK.
Methods: Primary SPK recipients were randomized to control or investigational arms as outlined in Figure 1. SPK recipients randomized to the investigational arm were eligible for CNI withdrawal at 40 weeks provided they had stable kidney and pancreas function, had no evidence of cellular and antibody mediated rejection during the first 280 days, and had no evidence of DSA at week 36 following SPK. Primary endpoint was estimated glomerular filtration rate (eGFR) at 52 weeks. Selected secondary endpoints included the incidence and severity of rejection, rates of insulin independence, HgbA1C, and safety measures. Autoantibody titers associated with Type I diabetes were tested at the time of transplant and monitored throughout the trial and at the time of CNI withdrawal. Pre-transplant frequency of CD4 57hi/PD1lo, a potential marker for ability to withdraw CNI, was measured at baseline in both groups.
Results: Twenty-one patients were randomized to the control arm; 22 to the investigational arm (Figure 1). 15/22 SPK recipients in the investigational arm were eligible for CNI withdrawal, and 11 completed withdrawal. SAE’s were common in both arms (Table 1). Renal function was equivalent in both groups. The high rate of pancreas AR following CNI withdrawal promoted the DSMB to stop CNI withdrawal. Autoantibody positivity at time of transplant for the entire cohort was 39.5% GAD65, 14.0% IA2, 72.1% MIAA and 14.0% ZNT8RW (Table 1). Although no general association between antiGAD65 titers and rejection events were detected in this cohort, there was a marked increase observed at the time of CNI withdrawal and rejection in one participant. No statistical difference was observed between pre-transplant frequencies of CD4 57hi/PD1lo T cells in rejectors versus non-rejectors.{{AbstractFigure.1}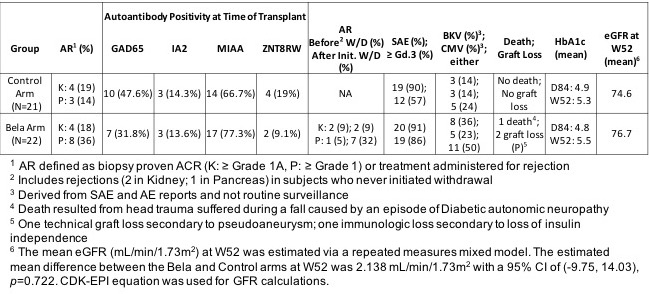
Conclusions: AR rates for both arms for both organs were low while maintained on either standard or low-dose CNI + bela. However, the high rates of pancreas AR in the bela arm following withdrawal of CNI demonstrate the importance of CNI in blocking the immune response following SPK. Belatacept may allow CNI minimization, but the high incidence of infection may outweigh long term benefits.
Future studies to identify biomarkers that characterize a cohort at increased risk of rejection may allow CNI withdrawal in a subset of recipients receiving belatacept.
