Casey J Ward, United States has been granted the TTS Young Investigator Scientific Award
Reversal of Diabetes and Preservation of Pancreatic Islet Grafts in the Extrahepatic Space with Novel Parathyroid Gland Co-Transplantation
Casey Ward1, Gaetano Faleo1, Quan Duh2, Mathias Hebrok3, Wenhan Chang4, Peter Stock1, Qizhi Tang1,3.
1Abdominal Transplant, UCSF, San Francisco, CA, United States; 2Endocrine Surgery, UCSF, San Francisco, CA, United States; 3Diabetes Research Center, UCSF, San Francisco, CA, United States; 4Endocrine Research, San Francisco Veterans Affairs, San Francisco, CA, United States
Islet transplantation can cure type 1 diabetes; however, multiple donors are needed to achieve insulin independence due to extensive perioperative loss of islets away from their native blood supply. In comparison, parathyroid gland (PTG) auto and allo-transplantation in the subcutaneous (SQ) and intramuscular (IM) sites is an established surgical procedure with great efficacy due to a unique resident CD34+ progenitor cell population. In this study, we aim to exploit the neoangiogenic and paracrine hormonal factors made by PTG derived CD34+ cells for preservation of human islet and stem cell derived beta cell co-transplantation in the SQ and IM.
Luciferase expressing B6 islets were co-transplanted with or without syngeneic mouse PTG in the SQ and IM and intervally imaged. Luciferase expressing Stem Cell derived Insulin Producing Cells (SCIPC.Luc) grafts with or without cryopreserved human PTG were co-transplanted in SQ and IM of NSG mice. CD34+ and CD34- cells were sorted using FACS and co-transplanted with SCIPC.Luc grafts in SQ. Mice were made diabetic using streptozocin (STZ).
Cotransplantation of PTG with both mouse islet and SCIPC in the SQ and IM resulted in complete preservation of islet graft in comparison to significant islet loss in mouse islet alone (10%-SQ, 20%-IM; Figure 1) or SCIPC alone (40%-SQ, 50%-IM; Figure 2) after 14 days (p <0.05). 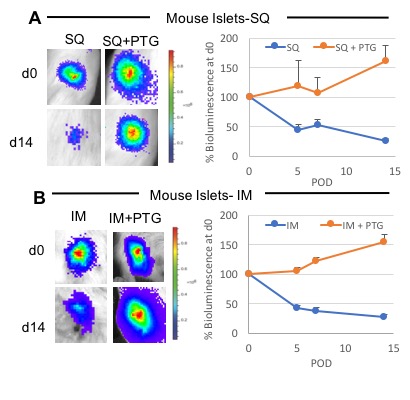
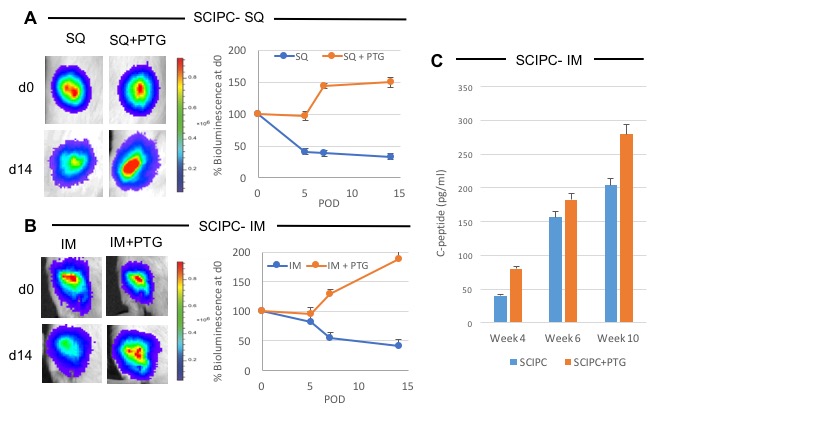 Furthermore, SCIPC+PTG co-transplanted grafts demonstrated significantly higher C-peptide levels compared to SCIPC alone grafts (Figure 2C). Cumulatively, this data demonstrates the ability of the PTG to preserve engraftment and islet function in mature and stem-cell derived islets. We further demonstrated this phenomenon with reversal of diabetes using suboptimal mature human islet mass (1000IEQ) in PTG cotransplanted diabetic mice in SQ. After 21 days, 75% of cotransplanted mice remained diabetes free compared to 0% of human islets alone (Figure 3).
Furthermore, SCIPC+PTG co-transplanted grafts demonstrated significantly higher C-peptide levels compared to SCIPC alone grafts (Figure 2C). Cumulatively, this data demonstrates the ability of the PTG to preserve engraftment and islet function in mature and stem-cell derived islets. We further demonstrated this phenomenon with reversal of diabetes using suboptimal mature human islet mass (1000IEQ) in PTG cotransplanted diabetic mice in SQ. After 21 days, 75% of cotransplanted mice remained diabetes free compared to 0% of human islets alone (Figure 3).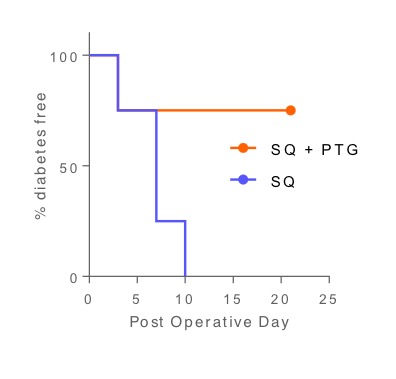
To elucidate the mechanism of this phenomenon further, we used FACS to isolate a novel CD34+ progenitor cell population within the PTG and performed cotransplantation of SCIPC with CD34+ versus CD34- cells. The SCIPC+CD34+ co-transplant recapitulated complete preservation of graft and improved C-peptide function compared to CD34- cotransplantation (Figure 4). 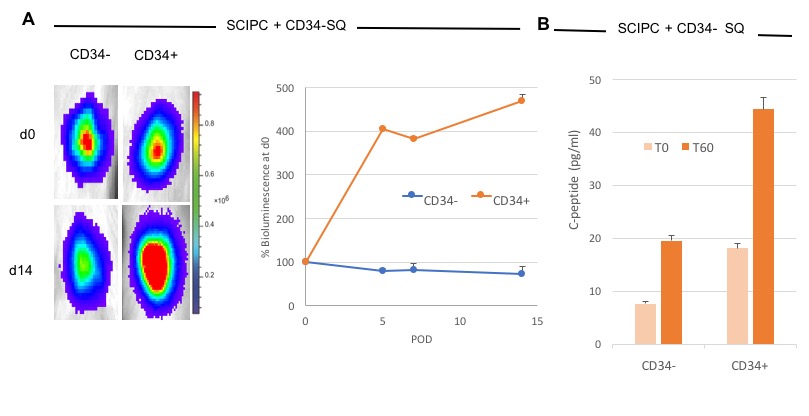
We show for the first time a novel co-transplantation of PTG with mature islets and SCIPC's leads to increased survival and function in the SQ and IM. Furthermore, the islet-protective activities of PTG resides in a small subpopulation of unique CD34+ progenitor cells. We believe the ability of the CD34+ progenitor cells to mature into endothelial cells, secrete pro-angiogenic and pro-survival hormonal factors cumulatively support mature islets and SCIPC's in foreign engraftment sites. The results of this study open many potential clinical trials in auto, allo, xeno or stem-cell based islet transplants for the future.
NIH FAVOR T32 Training Grant.
