
What's Normal? Using standardized flow cytometry immunophenotyping to create comprehensive pediatric reference ranges
Anne Halpin1,2,3,4,6, Morgan Sosniuk5, Juanita Wizniak4, Artur Szkotak3,4, Simon Urschel1,2,4,6, Lori J West1,2,3,4,5,6.
1Department of Pediatrics, University of Alberta, Edmonton, AB, Canada; 2Alberta Transplant Institute, University of Alberta, Edmonton, AB, Canada; 3Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada; 4Alberta Health Services, University of Alberta Hospital, Edmonton, AB, Canada; 5Department of Medical Microbiology and Immunology, University of Alberta, Edmonton, AB, Canada; 6Canadian National Transplant Research Program, N/A, N/A, AB, Canada
Introduction: Studying pediatric patients includes challenges such as difficulty obtaining samples, limited normal control reference data and small available sample volumes. Standardized flow cytometry immunophenotyping panels are commercially available to explore lymphocyte markers and extensive normal adult ranges have been published by the ONE Study group; comparable pediatric control data do not exist. Our objective is to establish a reference dataset to be available for pediatric studies using standardized, comprehensive flow cytometry panels with fresh whole blood. Our group had the opportunity to collaborate with the local clinical hematology laboratory to obtain normal pediatric samples with the goal of establishing comprehensive reference ranges across the pediatric age spectrum. This study also acted as a trial of this methodology for potential implementation in the clinical laboratory.
Methods: Using 700uL of EDTA blood, DuraClone IM (Beckman Coulter) flow cytometry phenotyping was performed on healthy pediatric controls ranging from 66 days to 16 years of age (n=10). Samples were tested in five, 10-colour flow cytometry T and B cell panels. Acquisition was performed on a Navios cytometer following calibration and standardization of cytometer output by Flow-Set™ Pro Fluorospheres. Analysis of the markers programmed cell death protein 1 (PD-1), often noted as a measure of T cell exhaustion, and gamma/delta (γδ) T cells and their associated markers Vd1 and Vd2, was performed.
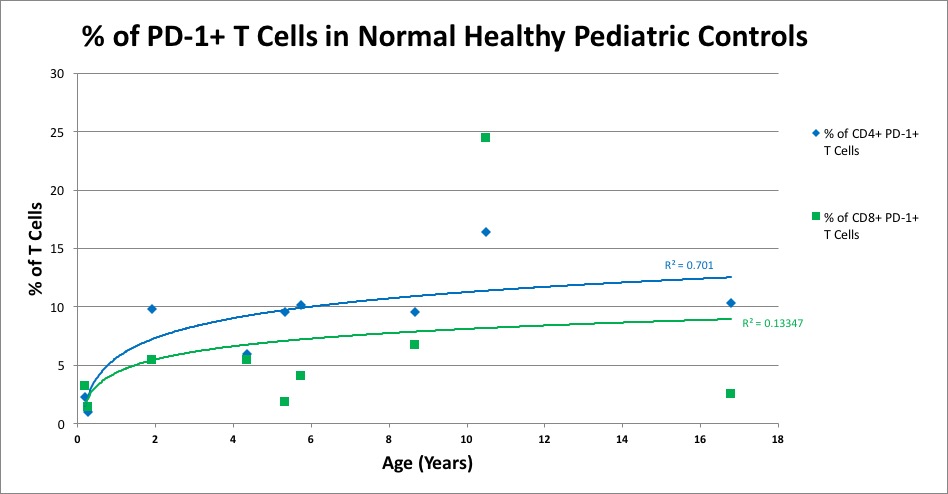
Results: Preliminary analyses show age related trends in PD-1 + T cells with increasing PD-1+ CD4 T cells with increasing age as shown in Figure 1 (R2=0.701); this association is much weaker for CD8 T cells. The number of CD3+ T cells, including γδ T cells, decreases with age, while the % of each population of cells remains constant. There is a trend indicating a decreasing ratio of V delta 1 to V delta 2 γδ T cells with age.
Conclusion: Duraclone is a rapid immunophenotyping method that requires small blood volumes. These results begin to establish valuable reference data for pediatric transplant patient populations and suggest that these flow panels show promise for application in the clinical flow cytometry laboratory due to their standardized nature. We will continue to explore age-related trends as we analyse additional data from these controls and test additional samples. Complete blood count data are available to calculate absolute cell counts and this analysis will be performed.
The use of fresh whole blood in this project differs from published pediatric reference range studies utilizing frozen cells and ensures populations are not lost or altered during mononuclear cell isolation or freeze thaw cycles. This study highlights the value of partnership between research and clinical laboratories as it can facilitate access to otherwise difficult to obtain samples and is valuable to the clinical laboratory in the exploration of new methodologies.
Women and Children's Health Reseach Institute (WCHRI).
