
AT1R Antibody Assessment: How Should We Interpret Positive Results?
Anne Halpin1,3,4,5, Sally Abou-Zeki3, Ingrid Larsen1,5, Patricia Campbell2,3,4,5, Simon Urschel1,4,5, Bruce Motyka1,5, Lori J West1,3,4,5.
1Department of Pediatrics, University of Alberta, Edmonton, AB, Canada; 2Department of Medicine, University of Alberta, Edmonton, AB, Canada; 3Department of Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada; 4Canadian National Transplant Research Program, N/A, N/A, AB, Canada; 5Alberta Transplant Institute, University of Alberta, N/A, AB, Canada
Introduction: The angiotensin II type 1 receptor (AT1R) is a G-protein-coupled receptor that is expressed on vascular endothelium. It can signal cell growth and proliferation, fibrosis, and vascular remodeling. AT1R antibodies are being increasingly studied to examine their relevance in transplantation. The frequency and impact of AT1R antibodies in pediatric heart transplantation has not been well studied. Our aim is to measure the frequency of AT1R antibodies in this patient population as well as pediatric controls and to assess for non-specific reactivity in this assay.
Methods: We included 42 patients (n=154 samples) for whom pre- and post-transplant sera and HLA antibody data were available. AT1R antibody levels were measured by a commercially available ELISA test (distributed by One Lambda ThermoFisher). Samples were tested/interpreted as per product insert. Results were negative under 10 U/mL and positive over 17 U/mL. Age-matched, sex-balanced, non-transplant controls (n=27) were collected from the local cardiac-catheterization laboratory. A subset of samples with positive results (n=52 sera from 20 patients) as well as the assay’s positive control were re-tested following adsorption with Adsorb Out (One Lambda ThermoFisher.). Figure 1 details the samples and testing methodology. Analysis was performed using GraphPad.
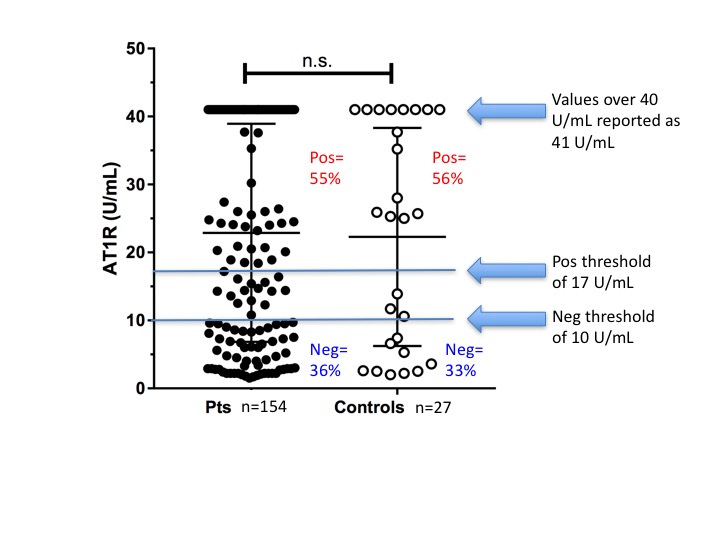
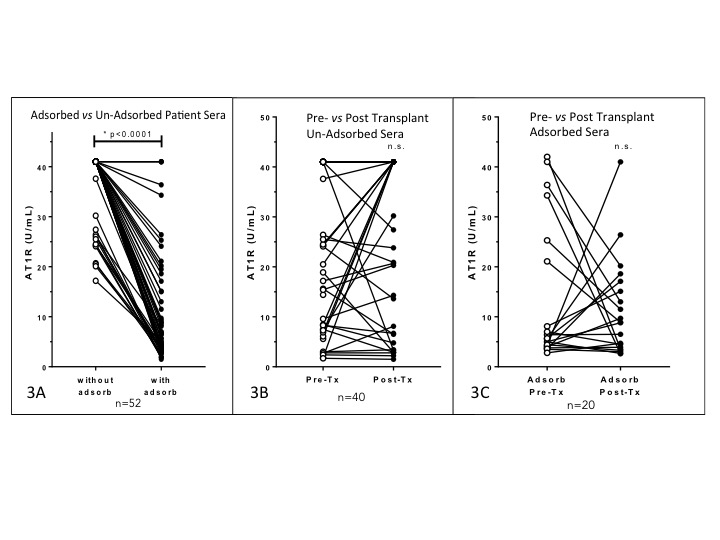
Results: No significant difference was observed between patient and control AT1R antibody levels (Figure 2). Values over the test method upper threshold (40 U/mL) were detected in 38% of patient sera. Adsorbed sera had significantly decreased AT1R values vs non adsorbed sera (Figure 3A). There was no significant change from pre- to post-transplant AT1R antibody detected overall (Figures 3B and 3C). In the UN-adsorbed sera, 57% of the pre-transplant sera and 64% of the post-transplant sera tested positive for AT1R antibody; 58% and 78% were positive above the 40U/mL threshold, respectively. In contrast, In the ADSORBED sera, 21% of the pre-transplant sera and 15% of the post-transplant sera tested positive for AT1R antibody; 29% and 20% were positive above the 40U/mL threshold, respectively.
Conclusion:Pediatric heart transplant patients appear to yield false positive results in this AT1R assay. This problem may be overcome by adsorbing for non-specific reactivity. Non-specific binding is a known concern in immunoassays such as ELISA. Typically, controls are included in these assays such as a ‘no antigen’ well and/or absorption steps are performed. This assay is lacking an essential control that is required by clinical laboratory standards. The pediatric heart transplant population may have especially high levels of non-specific reactivity in immunoassays due to the high frequency of thymectomy and mechanical circulatory support. We are now investigating the association of adsorbed and non-adsorbed AT1R antibody to transplant outcomes.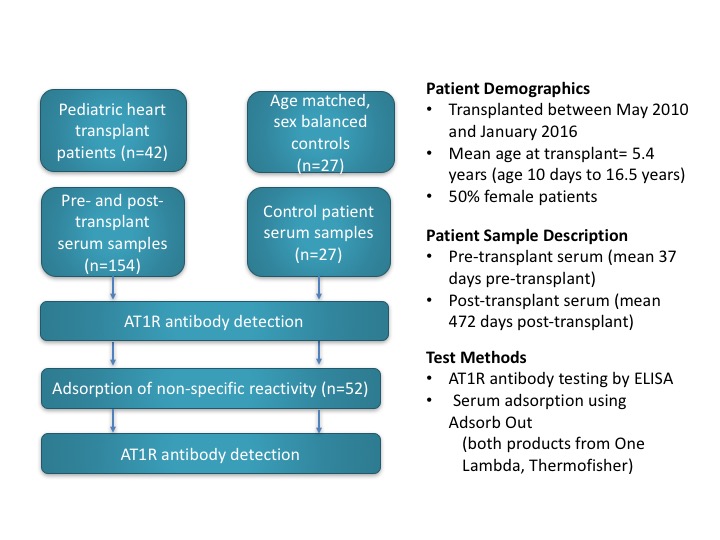
Canadian National Transplant Research Program (CNTRP). Edmonton Civic Employee’s Research Award.
