Histone Deacetylase-6 Inhibition is Protective in Liver Ischemia-Reperfusion Injury and Acetaminophen Toxicity in a Murine Model
Seth Concors1, Douglas Murken1, David D Aufhauser1, Zhonglin Wang1, Guanghui Ge1, Wayne W Hancock3,4, Matthew H Levine1,2.
1Department of Surgery, University of Pennsylvania, Philadelphia, PA, United States; 2Department of Surgery, Children’s Hospital of Philadelphia, Philadelphia, PA, United States; 3Department of Pathology and Laboratory Medicine, Children’s Hospital of Philadelphia, Philadelphia, PA, United States; 4Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA, United States
Introduction: Ischemia/reperfusion injury (IRI) is a major source of morbidity in liver transplantation and other surgical scenarios. In liver transplantation, IRI contributes to poor outcomes and early graft loss. A better understanding of the mechanisms of hepatic IRI may facilitate the development of new strategies for prevention and treatment. Histone deacetylases (HDACs) regulate diverse cellular processes. In the kidney, class I HDAC inhibition provides protection after ischemia. We sought to delineate the role of HDAC inhibition in mitigating liver ischemia reperfusion injury and other forms of liver injury.
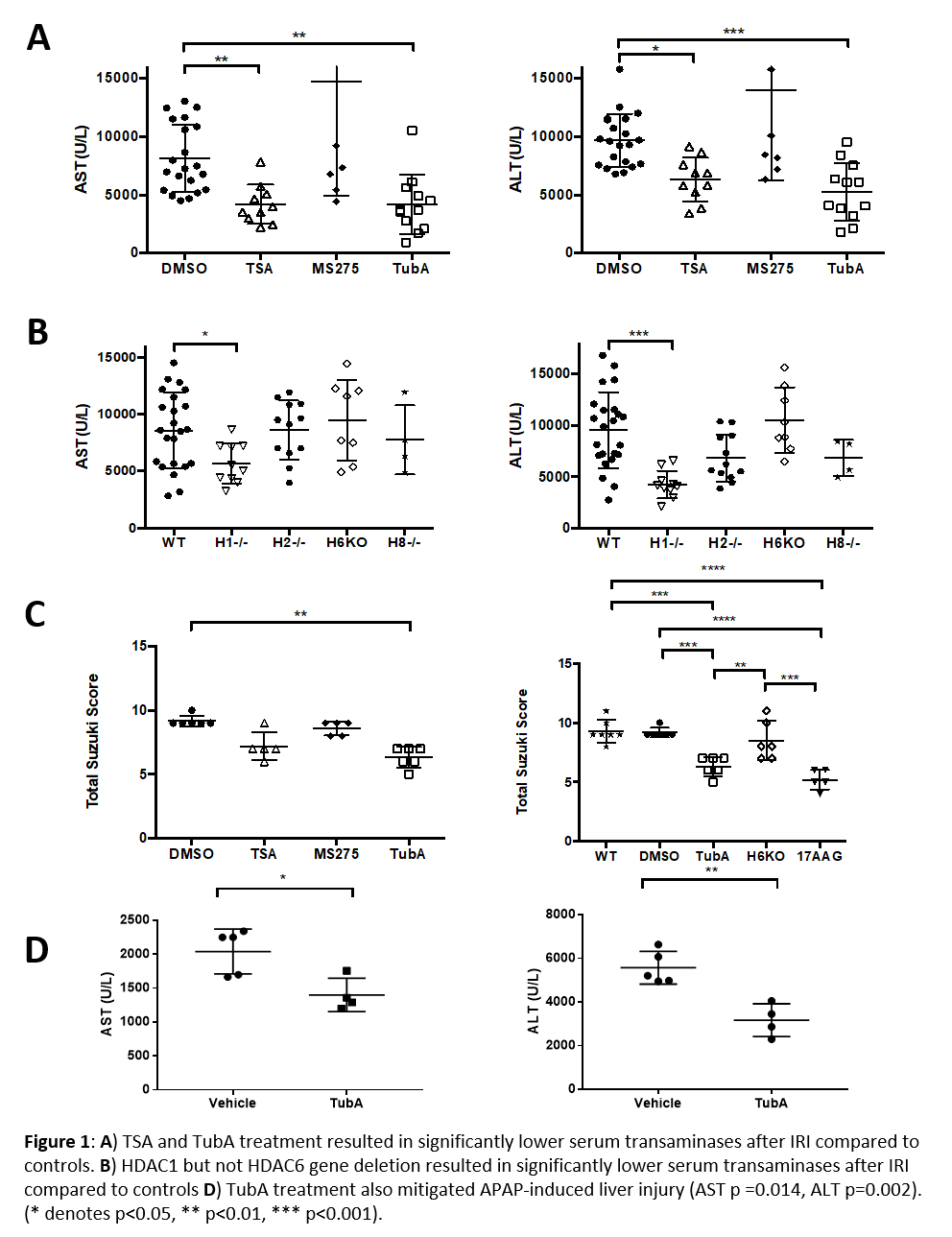
Methods: Male wild type C57BL/6 (WT) mice were treated with pan-HDAC inhibitor trichostain A (TSA), class I HDAC inhibitor MS-275, HDAC6 inhibitor Tubastatin-A (TubA), Hsp90 inhibitor (17AAG), or control vehicle (DMSO) at 16 and 1 hour pre-IRI. Whole-body tamoxifen-inducible HDAC-1, -2, -6, and -8 deficient and tamoxifen-treated WT male mice were also assessed. Mice were subjected to 70% liver ischemia for 60 minutes under strict temperature control. Histopathologic analysis was conducted at 24 hours post injury, and scored with the Suzuki Score for liver injury. In the acetaminophen (APAP) toxicity experiments, WT mice were treated with vehicle (Tween80, Peg500, and ddH20) or TubA at 16 hours and again just prior to administration of a sublethal dose of APAP (500mg/kg). In both experiments, AST and ALT levels were assessed 24 hours after injury.
Results: TSA- and TubA-treated mice developed significantly less hepatocellular injury after liver IRI than controls (Fig 1A). H1-/- mice developed significantly less injury after IRI compared to controls, but HDAC6 gene deletion did not mitigate IRI (Fig 1B). HDAC6 inhibition demonstrated decreased histologic liver injury (Figure 1C). TubA also mitigated APAP-induced hepatocellular injury (Fig 1D).
Conclusion: Pan-HDAC inhibition mitigates liver injury after IRI. Selective inhibition of HDAC1 via inducible gene deletion and pharmacologic inhibition of HDAC6 both replicate this protection. These findings are distinct from the pattern of HDAC involvement in renal IRI and suggest tissue-specific roles for HDACs in IRI response. Pharmacologic inhibition of HDAC6 also mitigated APAP-induced hepatocellular injury suggesting that the protective effects of TubA are not injury-type specific. Further experiments, including mechanistic studies, are required.
NIH/NIDDK 1K08DK092282-01.
