
Transgenic Expression of hCD47 Minimizes the Development of Proteinuria Following Pig-to-Baboon Kidney Transplantation and Reduces Phagocytosis of Porcine Endothelial Cells and Podocytes
Shunichiro Nomura1, Hironosuke Watanabe1, Yuichi Ariyoshi1, Thomas Pomposelli1, Gabriela Garcia2, Mitsuhiro Sekijima1, Lennan K Boyd1, Dilrukshi K Ekanayake-Alper1, Harrison C Glor1, Tatsu Tanabe1, Scott J Scott J Arn1, Robert J Hawley1, Megan Sykes1, David H Sachs1, Johnson J Richard 2, Kazuhiko Yamada1.
1Columbia Center for Translational Immunology (CCTI)/Surgery , Columbia University Medical Center, New York, NY, United States; 2Division of Renal Diseases and Hypertension, University of Colorado Denver, Aurora, CO, United States
Background: The early development of proteinuria following pig renal xenotransplantation (XKTx) was a major obstacle to prolonged life-supporting GalTKO XKTx in baboons. We have recently shown that CTLA-4Ig and Rituximab reduce proteinuria in association with downregulatation of the inflammatory response of CD80/SMPDL-3 in porcine glomeruli, allowing GalTKO thymokidneys (TK) without other transgenes (Tg) to function for >6 months. In this study, we addressed the hypothesis that failure of porcine CD47 to provide inhibitory signals to baboon macrophages via SIRPα triggers an inflammatory cascade that promotes proteinuria. We addressed this hypothesis by using hCD47 Tg porcine GalTKO animals as the source of TK.
Methods: Study 1 (in vivo study to assess the effect of hCD47 Tg on GalTKO TK donor on post-XKTx proteinuria): Two baboons received hCD47+/hCD55+ GalTKO kidneys (Group 1). One received hCD55+ GalTKO kidney (Group 2). As a control (Group 3), 6 baboons received GalTKO kidneys without additional Tg. All animals received an anti-CD154 mAb-based immunosuppressive regimen without CTLA4-Ig. Expression of hCD47 or hCD55 by donor kidneys was assessed histologically. All recipients had anti-non-Gal NAb antibody-complement mediated cytotoxicity lower than 35%. Study 2 (in vitro study): Expression of SIRPα and hCD47 in porcine glomeruli following XKTx was investigated. Phagocytosis of porcine endothelial cells (EC) as well as podocytes with/without hCD47 was assessed in co-culture assays.
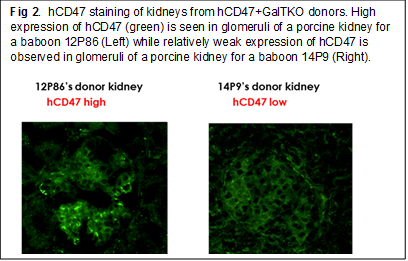
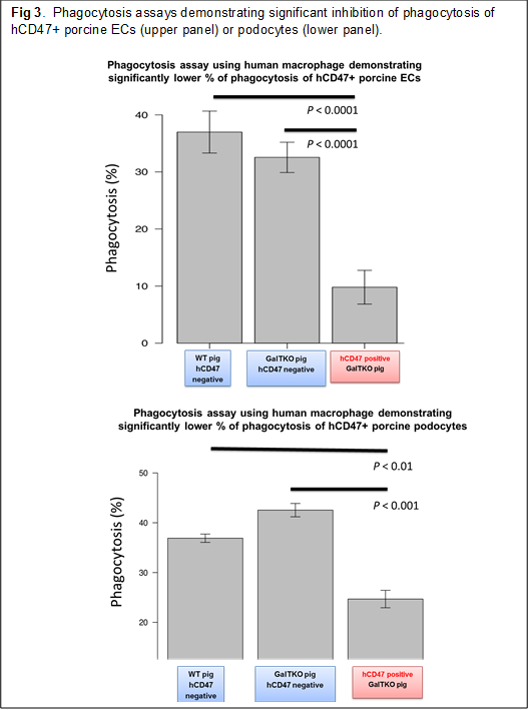
Results: Study 1: No elicited anti-pig ab developed following hCD47/CD55 Tg XKTx in any recipients. All of the recipients in Groups 2 and 3 had to be euthanized before POD70 in association with the development of massive proteinuria (Fig 1A-2, A-1). In contrast, of the two baboons in Group 1, the animal that expressed hCD47 highly in glomeruli (Fig. 2 left) developed only minimal proteinuria (>120 days). The other animal, which expressed hCD47 at a lower level (Fig 2 right) developed only 1+ and occasionally 2+ proteinuria and was euthanized at POD 69 due to graft growth (Fig.1A-3). Study 2: Expression of SIRPα on glomerular cells markedly decreased in GalTKO kidneys following XKTx, while no change in SIRPα was seen in hCD47+ GalTKO kidneys. Statistically significant reductions of phagocytosis of both porcine EC and podocytes were observed when hCD47 was expressed on porcine ECs (top in Fig 3) or podocytes (bottom in Fig 3).
Summary and Conclusions: These results demonstrate that (1) high expression of hCD47 on porcine glomerular cells in xenografts may prevent the development of proteinuria; and (2) the expression of hCD55 alone in kidneys does not prevent early post-XKTx proteinuria in these models. Taken together, these results suggest that interspecies incompatibilities between CD47 and SIRPα induce glomerular endothelial damage that penetrates the glomerular network and ultimately leads to disruption and damage of porcine podocytes.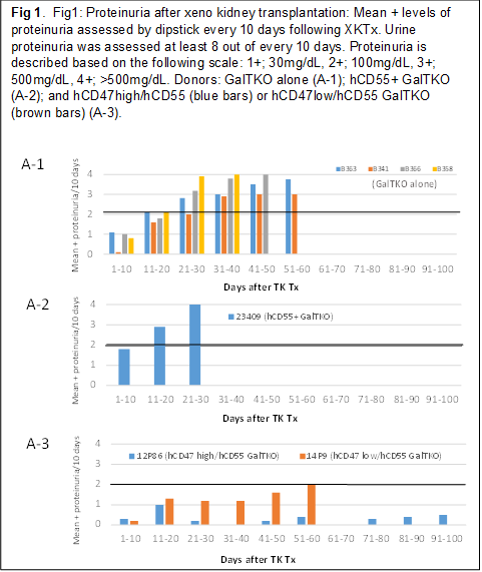
NIH grant P01AI45897.
