Efficacy of Direct Acting Antiviral Agents in Dialysis Patients with Chronic Hepatitis C Virus Infection on the Kidney Transplant Waiting List: a single center experience
Aysegul Oruc1, Abdulmecit Yıldız1, Tuba Öztürk2, Selim Gürel2, Suat Akgür1, Alparslan Ersoy1.
1Department of Nephrology, Uludağ University Medical School, Bursa, Turkey; 2Department of Gasroenterology, Uludağ University Medical School, Bursa, Turkey
Introduction: The incidence of hepatitis C virus (HCV) infection in chronic kidney disease (CKD) is higher than in the healthy population. Disadvantages such as side effects and inadequate response prevent the effective use of interferon and/or ribavirin treatments in dialysis patients. The risk of complications related to chronic HCV infection, incompatibility with conventional treatments, and prolonged waiting periods in the list are important problems for transplant candidates. Newly developed direct acting anti-viral agents (DAA) promise good results. In this study, we aimed to investigate the efficacy of DAA in HCV-RNA positive dialysis patients on kidney transplant waiting list in our center.
Method: A total 405 adult dialysis patients (mean age 49.9±13.8 years; 53.1% male) were listed on the national kidney transplant waiting list at our center. Among 26 anti-HCV positive patients on the list, 12 had HCV-RNA positivity. They were treated with DAA. The results at the end of the 12 week treatment, the frequency of adverse events and laboratory changes were assessed.
Results: The mean waiting time of 405 patients on the waiting list was 42.7±33.9 months. Anti-HCV positive candidates had longer waiting times than negative candidates (85.3±38.8 vs. 39.7±31.6 months, p<0.001). Fifteen transplant recipients had experience of interferon and/or ribavirin, and 7 could not complete the treatment for various reasons. In 9 patients who responded to treatment, HCV was reactivated in the follow-ups and sustained virologic response (SVR) could not be obtained.
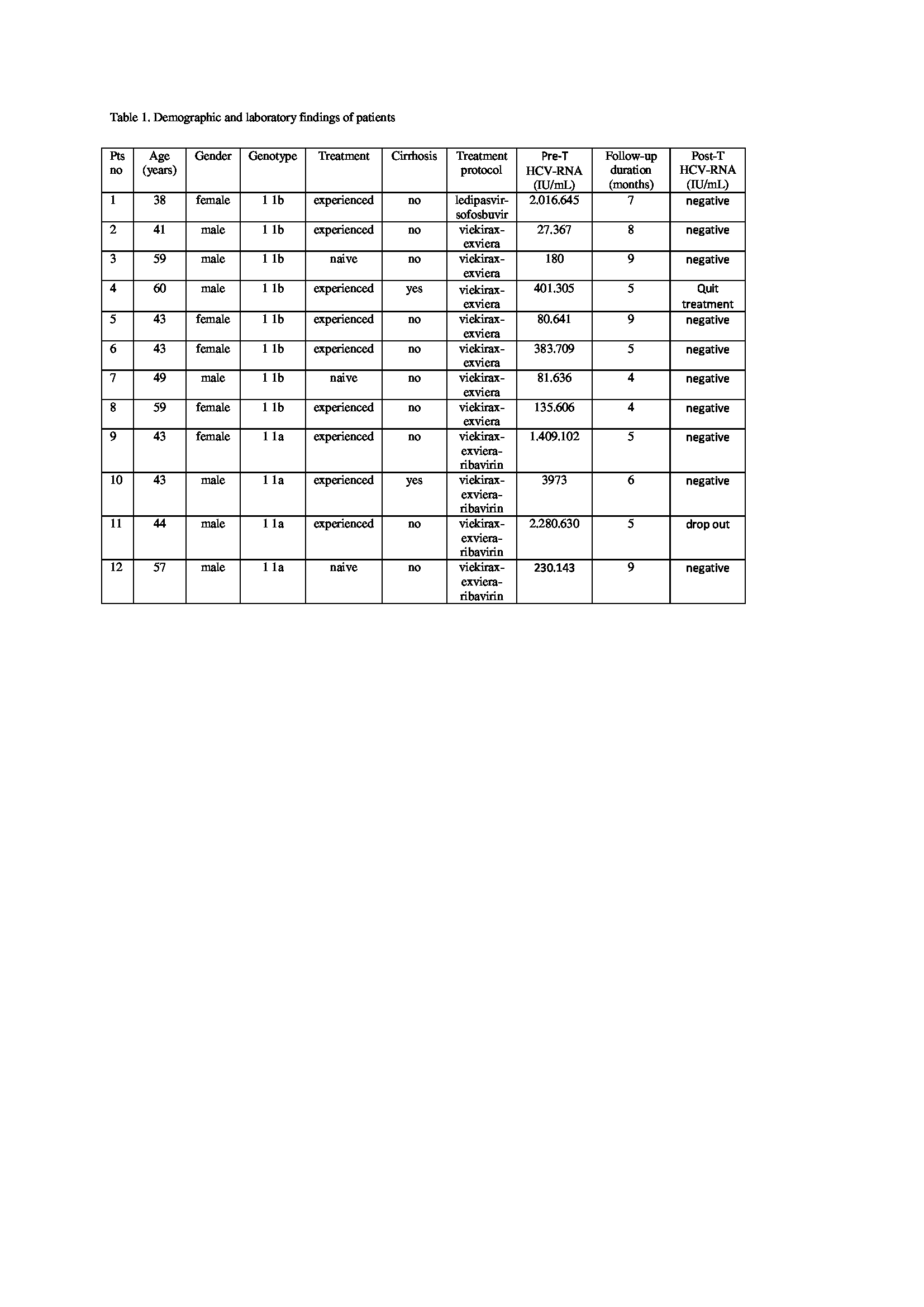
The DAA protocol (viekirax-exviera in 7, viekirax-exviera-ribavirin in 4, ledipasvir-sofosubvir in 1) was applied in 12 patients with HCV-RNA positive (genotype 1 1a in 4, genotype 1 1b in 8). Viekirax-exviera-ribavirin and viekirax-exviera were given for 12 weeks, ledipasvir-sofosuvir for 6 months. Two patient had cirrhosis. Three patients were treatment-naive, and 9 patients were treatment-experienced. The mean follow-up period of the patients was 6.33±1.96 months. HCV-RNA became negative in 10 of 12 patients after 12 weeks (Table 1). One patient was unable to complete the treatment with the cause of bleeding diathesis, and 1 patient dropped out from follow-up. There were no significant side effects associated with treatment in any patient.
Conclusion: HCV infection is an important cause of morbidity and mortality before and after kidney transplantation in CKD. It is recommended to obtain SVR before kidney transplantation. In our study HCV-RNA was negative in 83% of patients after 12 weeks. Our study supports that dialysis patients on the kidney waiting list should be treated with protocols that include DAA, which provides high SVR rates. However, long-term studies with more participants are required.