Recipient T Regulatory Cells Enhance Chimerism and Prolong Galt-KO pig Skin Graft Survival in Baboons
Nathaly Llore1, Jeffrey Stern1, Paula Alonso-Guallart1, Karl E Berglund1, Alina Luga 2, Josh Weiner 1, Genevieve Pierre1, Emily Lopes 1, Samanta Gokova1, David Ayares3, Marc Lorber4, Robert Hawley1, Kazuhiko Yamada1, David Sachs1, Megan Sykes 1, Adam Griesemer1.
1Columbia Center for Translational Immunology, Columbia University, New York, NY, United States; 2Department of Pathology and Cell Biology, Columbia University, New York, NY, United States; 3Revivicor, Revivicor , Blacksburg, VA, United States; 4United Therapeutics, United Therapeutics, Silver Spring , VA, United States
Introduction: Organ availability continues to be one of the most challenging obstacles in the field of transplantation. While pigs may be a viable organ source given their physiologic compatibility to humans, the strong immune response across xenogeneic barriers is still limiting. The use of GalTKO pigs has eliminated a main target for the recipient antibody response. We have previously evaluated the transplantation of mobilized GalTKO/ hCD46/ hCD47 pig PBSCs to baboon recipients to induce xenogeneic chimerism and reported marked prolongation of donor skin grafts. We have now examined whether chimerism can be further enhanced by the use of polyclonal expanded recipient Tregs
Methods: Eight P. hamadryas received a non-myeloablative preconditioning regimen consisting of total body irradiation, thymic irradiation, rituximab, ATG, anti-CD154, anti-CD2 antibody and rapamycin. This regimen was followed by infusion of cytokine-mobilized GalTKO/ hCD46/ hCD47 pig PBSCs in conjunction with ex-vivo expanded autologous Tregs at three time points. 6 of 8 baboons received Tregs. Immunosuppression was completely tapered completely before donor pig skin transplantion (day 90-100 after initital PBSC/Treg infusion). Peripheral blood and bone marrow chimerism were detected by flow cytometry. Anti-pig responses were detected by mixed lymphocyte reaction (MLR) and anti-donor serum antibody levels.
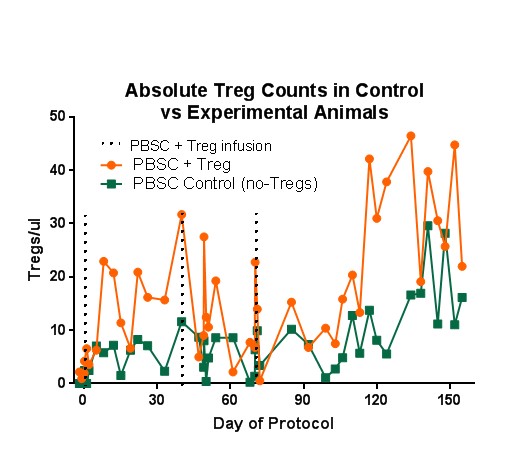
Results: Macrochimerism was higher and persisted longer in baboons receiving Treg infusions. An initial increase in absolute numbers of Tregs was associated with each porcine PBSC/Treg infusion on days 0, 49, and 70, in comparison to the control group that did not receive Tregs (Figures 1 and 2). Baboons that received Tregs had markedly prolonged donor pig skin graft survival ranging from 16 to 30 days, which was longer than survival on the control animals, as determined by appearance and histology.
Conclusions: The presence of hCD47 on mobilized pig PBSC leads to macrochimerism and prolonged donor skin graft survival on conditioned baboons. The administration of ex vivo expanded autologous Tregs further prolongs donor chimerism and donor skin graft survival. 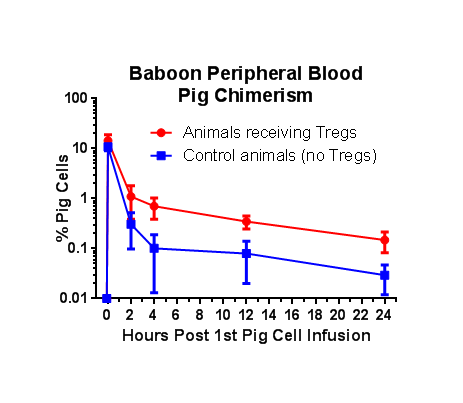
United Therapeutics.
