High Fibre Diet Induces Donor Specific Tolerance of Kidney Allografts through SCFA Induction of Tregs
Huiling Wu1,2, Tony Kwan2, Yik Wen Loh2, Chuanmin Wang1,2, Laurence Macia2,3, Stephen Alexander2,4, Steven Chadban1,2.
1Kidney Node Laboratory, The Charles Perkins Centre and Renal Medicine, Royal Prince Alfred Hospital, Sydney, Australia; 2Sydney Medical School, The University of Sydney, Sydney, Australia; 3Nutritional Immunometabolism Lab, The Charles Perkins Centre, Sydney, Australia; 4Centre for Kidney Research, The Children Hospital at Westmead, Sydney, Australia
Short-chain fatty acids (SCFAs) are derived from gut microbial fermentation of dietary fibre and are thought to regulate gut and systemic immunity through mechanisms including promotion of Tregs. The molecular actions of SCFAs involve the activation of ‘metabolite-sensing’ cell-surface G-protein-coupled receptors (GPCRs) such as GPR43 and GPR109a. High fibre diet or dietary delivery of SCFAs (eg. acetate, butyrate and propionate) have been found to manipulate intestinal microbiota and attenuate the development of experimental autoimmune and inflammatory diseases. Here we examined the impact of high fibre (HF) diet or dietary supplementation with the SCFA sodium acetate (SA) on kidney allograft rejection and survival in a fully MHC-mismatched murine model of kidney transplantation.
Methods: Life-sustaining kidney transplants were performed: BALB/c to B6, GPR43-/- and GPR109a-/- mice as allografts, and B6 to B6 (isografts). Mice were fed HF diet for 2 weeks prior and throughout experiments (WT+HF), or received 200mg/kg SA ip for 14 days post-transplantation, followed by oral 150mM SA solution (WT+SA; GPR43-/-+SA; GPR109a-/-+SA). Allograft control group received normal chow only (WT allografts). To deplete CD4+CD25+ cells in vivo, selected groups received anti-CD25 mAb (PC61). Experiments included: (1) survival study to 100 days; (2) Histological and functional assessment of acute and chronic allograft rejection responses in SA treated groups and controls sacrificed on days 14 and 100.
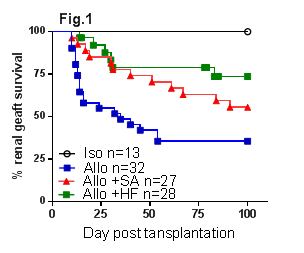
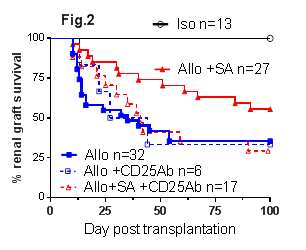
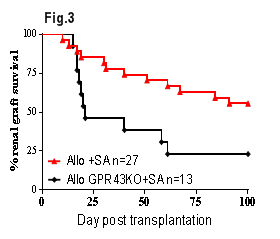
Results: WT+HF allografts had significantly prolonged survival compared to WT allografts (Fig.1, p<0.01). HF feeding increased the release of SCFAs, particularly acetate. Similarly, WT+SA allografts were protected from both acute and chronic kidney allograft rejection with better renal function (p<0.05), less tubulitis (p<0.001) and increased CD4+Foxp3+ Treg accumulation at day 14 post-transplant, and improved renal function (p<0.05) and less proteinuria (p<0.001) at day 100 post-transplant versus WT allografts. WT+SA allografts exhibited superior survival to WT controls (Fig.1, p<0.05) due to the development of donor antigen specific tolerance, confirmed by acceptance of donor strain but rejection of 3rd party skin grafts. The survival benefit conferred by SA was broken by depletion of CD25+ Tregs (Fig.2, p<0.05). SA treatment was ineffective in GPR43-/- allograft recipients (GPR43-/-+SA, Fig.3, p<0.05). Studies of SA treatment of GPR109a-/- allograft mice (GPR109a-/-+SA) are underway.
Conclusions: HF diet or dietary supplementation with SA induced donor specific kidney allograft tolerance in a fully MHC mismatched murine model of kidney allograft rejection. Tolerance was dependent on a CD4+CD25+FoxP3+ regulatory mechanism. GPR43 is required for the molecular action of SA induced donor specific kidney allograft tolerance.