
Tomotherapy and Hematopoietic Stem Cells for Tolerance to Kidney Transplants in Rhesus Macaques
Dixon B. Kaufman1, Lynn Haynes1, Will Burlingham1, Peiman Hematti1, Lisa Forrest2, Jen Post1, Ewa Jankowska-Gan1, Kent Jensen3, Samuel Strober3.
1Surgery/Medicine, University of Wisconsin, Madison, WI, United States; 2Veterinary Medicine, University of Wisconsin, Madison, WI, United States; 3Medicine, Stanford University, Palo Alto, CA, United States
NIH NHP Transplantation Tolerance Cooperative Study Group.
The goal of this project was to develop a tolerance induction protocol for kidney transplantation (tx) in a rhesus macaque model that would permit complete elimination of all immunosuppression (IS). We hypothesized that tolerance to MHC 1-haplotype mismatched kidney tx between living related donor/recipient pairs can be achieved by establishing a mixed chimeric state. The chimeric state would be achieved using a newly established post-kidney tx, non-myeloablative, helical tomotherapy-based total lymphoid irradiation (TLI)-based conditioning regimen followed by donor mobilized peripheral blood-CD34+ hematopoietic stem cell (HSC)/T cell (CD3+) infusions (Figure 1).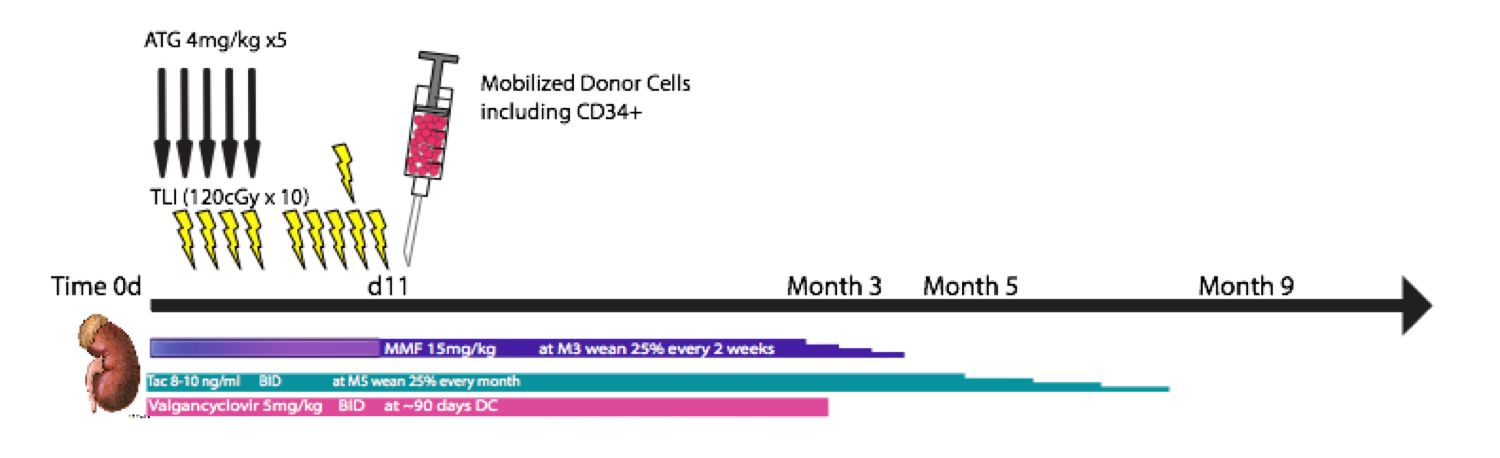 The hypotheses was tested in a series of 5 HSC-only txs followed by 18 kidney txs with (n=11) and without (n=7) donor HSC infusions. The kidney allografts came from blood group compatible, T and B cell flow cytometric crossmatch negative donors. All recipient animals underwent IS withdrawal according to the same schedule and were followed for rejection. We determined the proportion of recipients that achieved chimerism and that could be withdrawn from all IS for greater than 3 years, while maintaining normal allograft function. Mixed chimerism in the recipient was assessed by quantifying the proportion and duration of donor/recipient DNA, using STR, in various immune cellular lineages circulating within the peripheral blood post-tx. Results are shown in Figure 2.
The hypotheses was tested in a series of 5 HSC-only txs followed by 18 kidney txs with (n=11) and without (n=7) donor HSC infusions. The kidney allografts came from blood group compatible, T and B cell flow cytometric crossmatch negative donors. All recipient animals underwent IS withdrawal according to the same schedule and were followed for rejection. We determined the proportion of recipients that achieved chimerism and that could be withdrawn from all IS for greater than 3 years, while maintaining normal allograft function. Mixed chimerism in the recipient was assessed by quantifying the proportion and duration of donor/recipient DNA, using STR, in various immune cellular lineages circulating within the peripheral blood post-tx. Results are shown in Figure 2.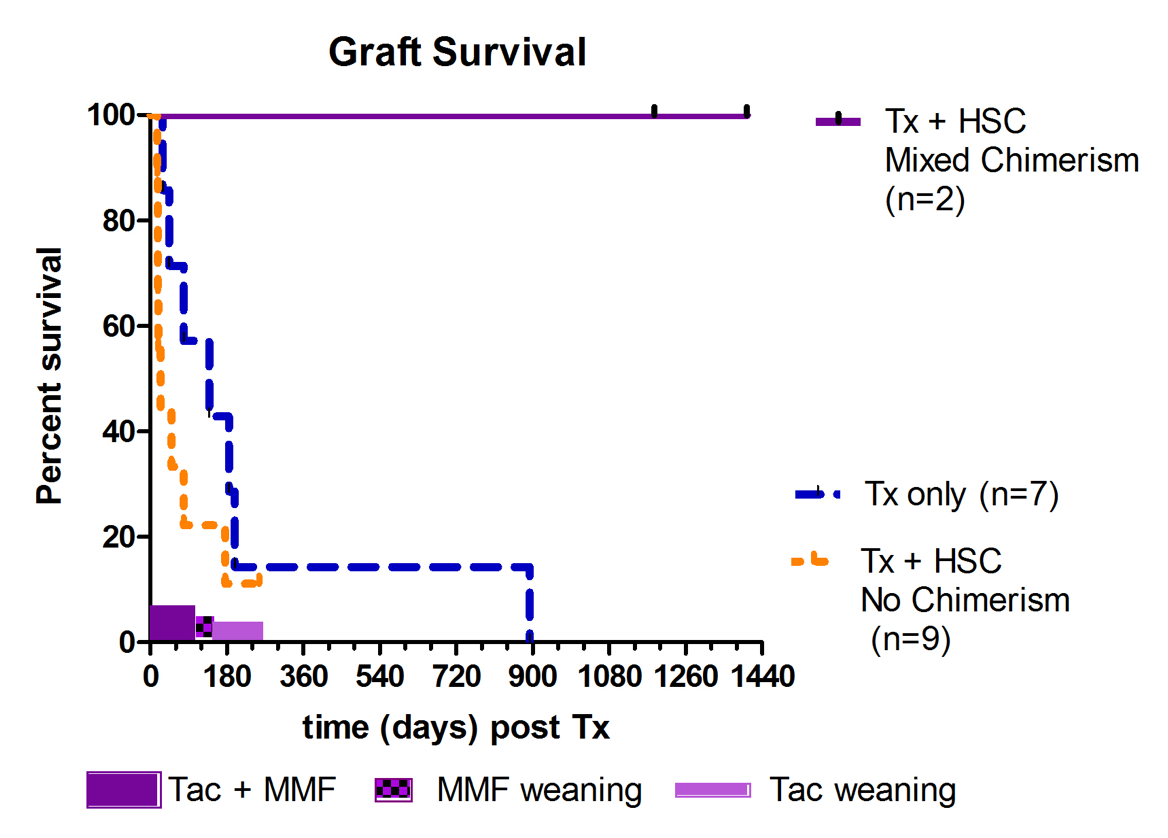 There were 11 kidney+HSC txs. Two achieved transient multilineage chimerism for at least 3 months and both were long-term tolerant survivors (still alive and off IS for more than 3 years). Nine animals in the kidney+HSC group did not achieve any evidence of chimerism -- all lost the kidney tx before or during the IS weaning (5 due to rejection, 4 with engraftment syndrome, 1 each from infection and PTLD). All animals in the kidney tx-alone control group lost kidney function, with one having prolonged graft survival without IS, but eventually succumbed to chronic antibody mediated rejection. Furthermore, we assessed the correlation between the presence (n=5, including 3 with HSC-only infusions) or absence (n=8, including 2 with HSC-only infusions and excluding the 4 engraftment syndrome cases) of achieving chimerism and the development of de novo donor specific antibody (DSA) (Figure 3).
There were 11 kidney+HSC txs. Two achieved transient multilineage chimerism for at least 3 months and both were long-term tolerant survivors (still alive and off IS for more than 3 years). Nine animals in the kidney+HSC group did not achieve any evidence of chimerism -- all lost the kidney tx before or during the IS weaning (5 due to rejection, 4 with engraftment syndrome, 1 each from infection and PTLD). All animals in the kidney tx-alone control group lost kidney function, with one having prolonged graft survival without IS, but eventually succumbed to chronic antibody mediated rejection. Furthermore, we assessed the correlation between the presence (n=5, including 3 with HSC-only infusions) or absence (n=8, including 2 with HSC-only infusions and excluding the 4 engraftment syndrome cases) of achieving chimerism and the development of de novo donor specific antibody (DSA) (Figure 3).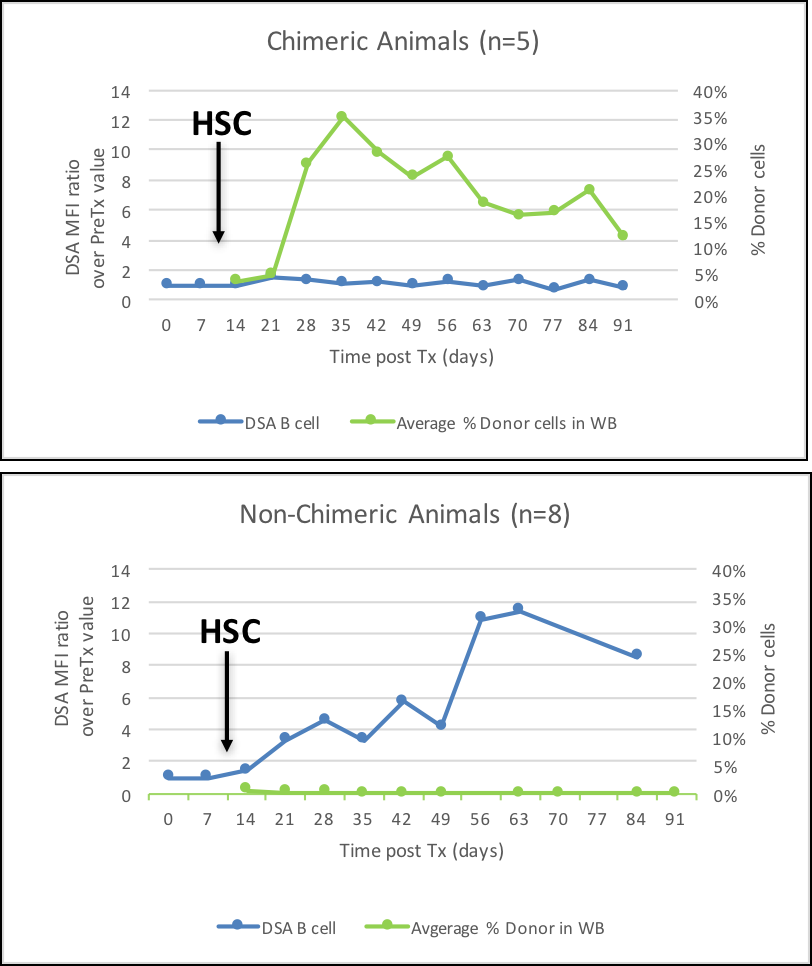 DSA was absent in all 5 animals that achieved chimerism. Development of early de novo DSA, primarily against Class II MHC, was associated with lack of development of mixed chimerism, and therefore, inability to successfully eliminate IS. The study demonstrated that this TLI-based tolerance induction protocol can result in chimerism and operational tolerance in the rhesus macaque model across a 1-haplo mismatch. However, challenges remain to enhance the frequency of achieving chimerism. Future studies to extend the protocol to animals that are more MHC disparate will include modifications of the conditioning regimen to minimize development of DSA and engraftment syndrome.
DSA was absent in all 5 animals that achieved chimerism. Development of early de novo DSA, primarily against Class II MHC, was associated with lack of development of mixed chimerism, and therefore, inability to successfully eliminate IS. The study demonstrated that this TLI-based tolerance induction protocol can result in chimerism and operational tolerance in the rhesus macaque model across a 1-haplo mismatch. However, challenges remain to enhance the frequency of achieving chimerism. Future studies to extend the protocol to animals that are more MHC disparate will include modifications of the conditioning regimen to minimize development of DSA and engraftment syndrome.
Funding supported by NIH NIAID.
