Long-Term Nonhuman Primate Renal Allograft Survival without Ongoing Immunosuppression in Recipients of Delayed Donor Bone Marrow Transplantation
Kiyohiko Hotta1, Tetsu Oura1, Abbas Dehnadi1, Masatoshi Matsunami1, Ivy Rosales1, Rex-Neal Smith1, Robert B Colvin1, A. Benedict Cosimi1, Tatsuo Kawai1.
1Transplant Center, Massachusetts General Hospital, Boston, MA, United States
Background: We have previously reported induction of renal allograft tolerance in nonhuman primates following an initial post-transplant period of conventional immunosuppression (IS)(delayed tolerance) using a nonmyeloablative conditioning regimen that included anti-CD154 and anti-CD8 mAbs plus horse ATG (hATG) and donor bone marrow transplantation (DBMT). Since these reagents are not currently clinically available, the protocol has been revised to be applicable to human recipients of deceased donor allografts.
Method: All recipients initially underwent KTx alone with conventional IS. Four months later, the recipients underwent conditioning and DBMT. Group 1 recipients received low-dose total body irradiation (TBI; 1.5Gy x 2), thymic irradiation (TI; 7 Gy), and horse ATG. After DBMT, anti-CD154 mAb for 2 weeks and cyclosporine A for one month were administered after which no further immunosuppression was administered. In Group 2, anti-CD8 mAb was added to the Group 1 regimen. In Group 3, hATG, anti-CD8 mAb, and anti-CD154 mAb were replaced with rabbit-ATG (Thymoglobulin) and CTLA4Ig (belatacept).
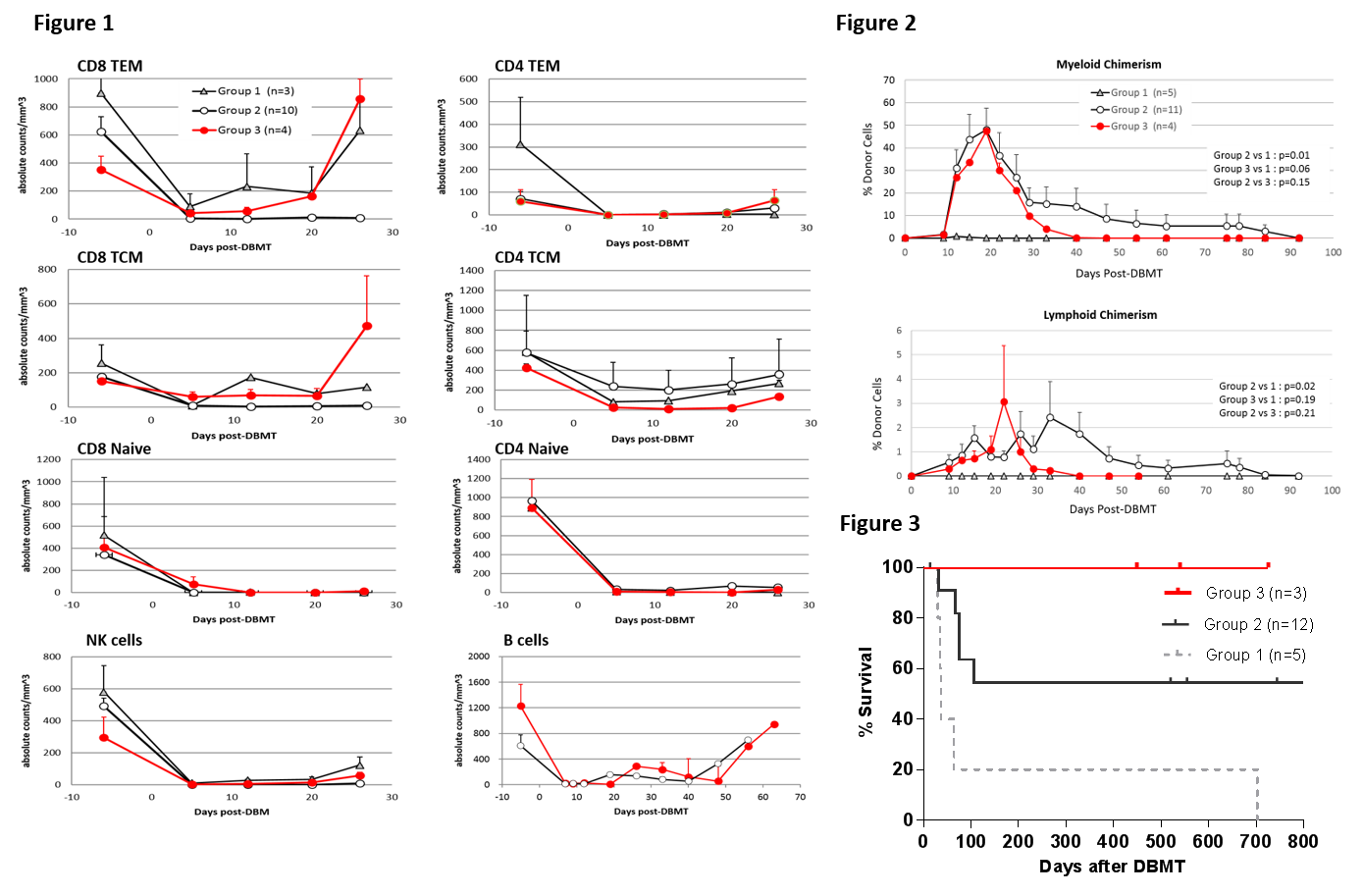
Results: With the Group 1 regimen, rapid recovery of CD8+ effector memory T cells (TEM) and central memory T cells (TCM) was observed by day 12 (Fig 1, triangle), along with minimal induction of mixed chimerism (Fig 2, triangle). 4/5 recipients in this group failed to achieve long-term survival (Fig.3). Adding anti-CD8 mAb to the Group 1 regimen extended the duration of CD8+ TEM and TCM deletion to more than 30 days (Fig. 1, open circle), and 10/12 successfully developed mixed chimerism (Fig 2. right panel, open circle). However only 6/12 achieved long-term survival because of death due to viral infection and PTLD (Fig.3). With the Group 3 regimen, the deletion of CD8+ TEM and TCM was less intense than that observed in Group 2, but deletion of CD8+ TEM was superior to that in Group 1 recipients until day 20 (Fig. 1, red circle). This regimen, nevertheless, provided successful induction of chimerism in four consecutive recipients (Fig. 2, red circle). Three recipients achieved long-term IS free renal allograft survival without infectious complication or PTLD (one died due to anesthesia complication during a biopsy procedure without rejection).
Conclusion: This study provides proof of principle that induction of mixed chimerism and long-term renal allograft survival without IS after delayed donor bone marrow transplantation is possible with clinically available reagents.