Induction of Tolerance for Long-Term Liver Allograft Survival Without Immunosuppression in Cynomolgus Monkeys
Erik Berglund1, Sulemon Chaudhry1, Yojiro Kato1, Joshua Weiner1, Paula Alonso-Guallart1, Nathaly Llore1, Jeffrey Stern1, Dilrukshi Ekanayake-Alper1, Mercedes Martinez2, Genevieve Pierre1, Makenzie Danton1, Sigal Kofman1, Diane Ordanes1, Jay H. Lefkowitch3, Alina Iuga1, Tomoaki Kato4, Megan Sykes1, Adam Griesemer1.
1Columbia University, Columbia Center for Translational Immunology, New York, NY, United States; 2Department of Pediatrics, College of Physicians and Surgeons, Columbia University, New York, NY, United States; 3Departments of Pathology and Cell Biology, College of Physicians and Surgeons, Columbia University, New York, NY, United States; 4Department of Surgery, Center for Liver Disease and Transplantation, Columbia Presbyterian Hospital, Columbia College of Physicians and Surgeons, New York, NY, United States
Background: Spontaneous liver tolerance occurs only in highly selected patients with stable liver function for several years. There is an unmet need for a safe and reliable regimen allowing rapid immunosuppression (IS) withdrawal. Herein, we investigated if tolerance to liver allografts can be achieved in cynomolgus macaques using four different non-myeloablative preparative regimens.
Methods: Nine combined liver and bone marrow cell transplants were performed in cynomolgus monkeys, using three various conditioning regimens (group A, B, C). All animals received total body irradiation (1.5 Gy), thymic irradiation (7 Gy), and ATGAM (50 mg/kg) prior to the transplant. Rituximab (20 mg/kg), anti-CD40 (50 mg/kg), and anti-CD2 (1-2 mg/kg) monoclonal antibodies were given according to Table 1. Four additional livers (group D) were transplanted with the same protocol as in group C, but BMT was omitted. All IS was completely discontinued by day 55.
Results: All recipients developed multilineage mixed chimerism. Group A showed severe rejection with CD8+ T effector memory (Tmem) cell expansion. Improved pretransplant host Tmem depletion using anti-CD2 in group B resulted in prolonged multlineage MC and graft acceptance. Animal B(2), however, developed grade 1 GVHD in skin and rectum. The addition of post-transplant anti-CD2 in group C depleted donor Tmem, prevented GVHD, while permitting prolonged MC and extended IS-free graft survival. With the exclusion of BMT (group D), passenger leukocytes in the liver promoted transient MC. Even when chimerism was lost and memory T cells recovered, group D maintained normal graft function without any inflammation seen in protocol biopsies beyond six months. The percentage of Tregs in total CD4 were elevated over baseline during the period of stable graft function in Groups C and D animals.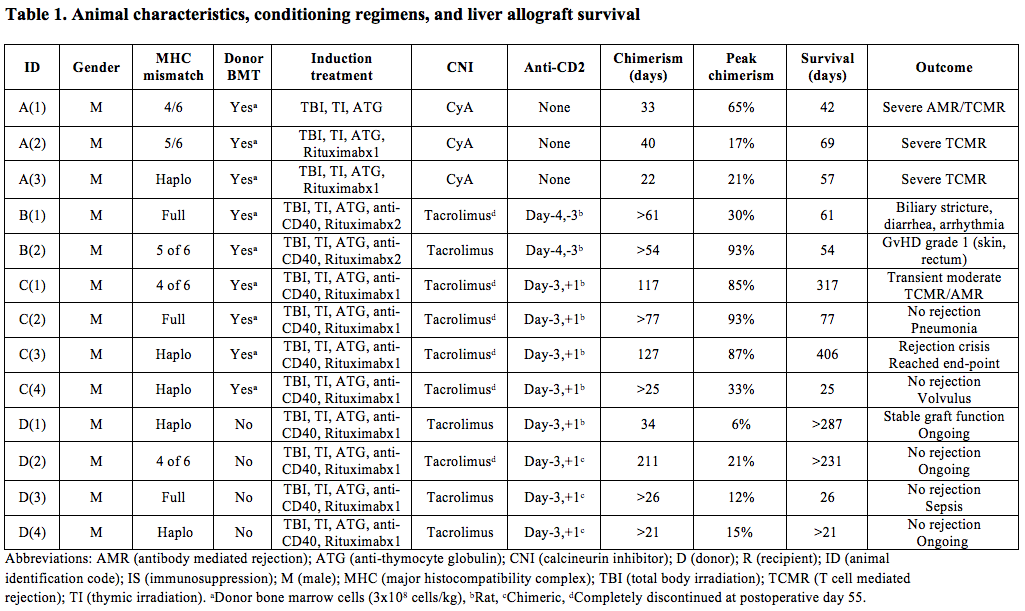
Conclusions: This is the first proof of concept study demonstrating long-term stable liver allograft function in nonhuman primates using a non-myeloablative treatment regimen to facilitate rapid IS withdrawal. The results demonstrate the ability of anti-CD2 to control Tmem responses, and that liver transplantation alone (without infusion of DBM cells) generates MC and is associated with long-term graft survival after complete IS withdrawal.
NIAID R56AI122332-01A1 . AASLD Career Development Award in the Memory of the University of Michigan Transplant Team. Columbia University Irving Institute for Clinical Research.
