Intimal Arteritis Associated with Microvascular Inflammation even in the Absence of Donor-Specific Antibodies might result into early Chronic Rejection
Marek Novotny1, Petra Hruba2, Petra Vichova3, Eva Honsova4, Ondrej Viklicky1,2, Mariana Wohlfahrtova1,2.
1Nephrology, Institute for Clinical and Experimental Medicine, Prague, Czech Republic; 2Transplant Laboratory, Institute for Clinical and Experimental Medicine, Prague, Czech Republic; 3Department of Immunogenetics, Institute for Clinical and Experimental Medicine, Prague, Czech Republic; 4Department of Clinical and Transplant Pathology, Institute for Clinical and Experimental Medicine, Prague, Czech Republic
Background: According to recent Banff classification intimal arteritis (v-lesion) is a histological feature of T cell-mediated (TCMRV) and antibody-mediated vascular rejection (AMRV). AMRV has been recognized as a predictor of poor kidney allograft survival exceeding other rejection phenotypes. Thorough analysis of histological features is needed in order to avoid misclassification of acute vascular rejection (AVR) phenotypes with potentially deleterious impact on graft survival.
Materials and Methods: In single centre prospective observational study (May 2015-October 2017) patients with newly diagnosed AVR within 1 year after kidney transplantation were consented to close follow-up including surveillance biopsy and assessment of donor-specific antibodies (DSA) 3 months after diagnosis.
Results: 46 patients were included into study. 10 patients experienced TCMRV defined as v-lesion accompanied with tubulointerstitial inflammation with no signs of microvascular inflammation (MI) or DSA. Other 10 patients experienced AMRV defined as v-lesion, MI and/or C4d positivity and detectable DSA. Remaining 26 patients present with MI and/or C4d positivity but no detectable DSA. These patients were classified as suspected antibody-mediated vascular rejection (sAMRV). Rejection persistence was observed in surveillance biopsies of 3 (30%) TCMRV, 10 (38%) sAMRV and 8 (80%) AMRV (p=0.004). Transplant glomerulopathy (cg) was described exclusively in surveillance biopsies of sAMRV (n=8, 31%) and AMRV (n=8, 80%) (p=0.004) (Figure 1).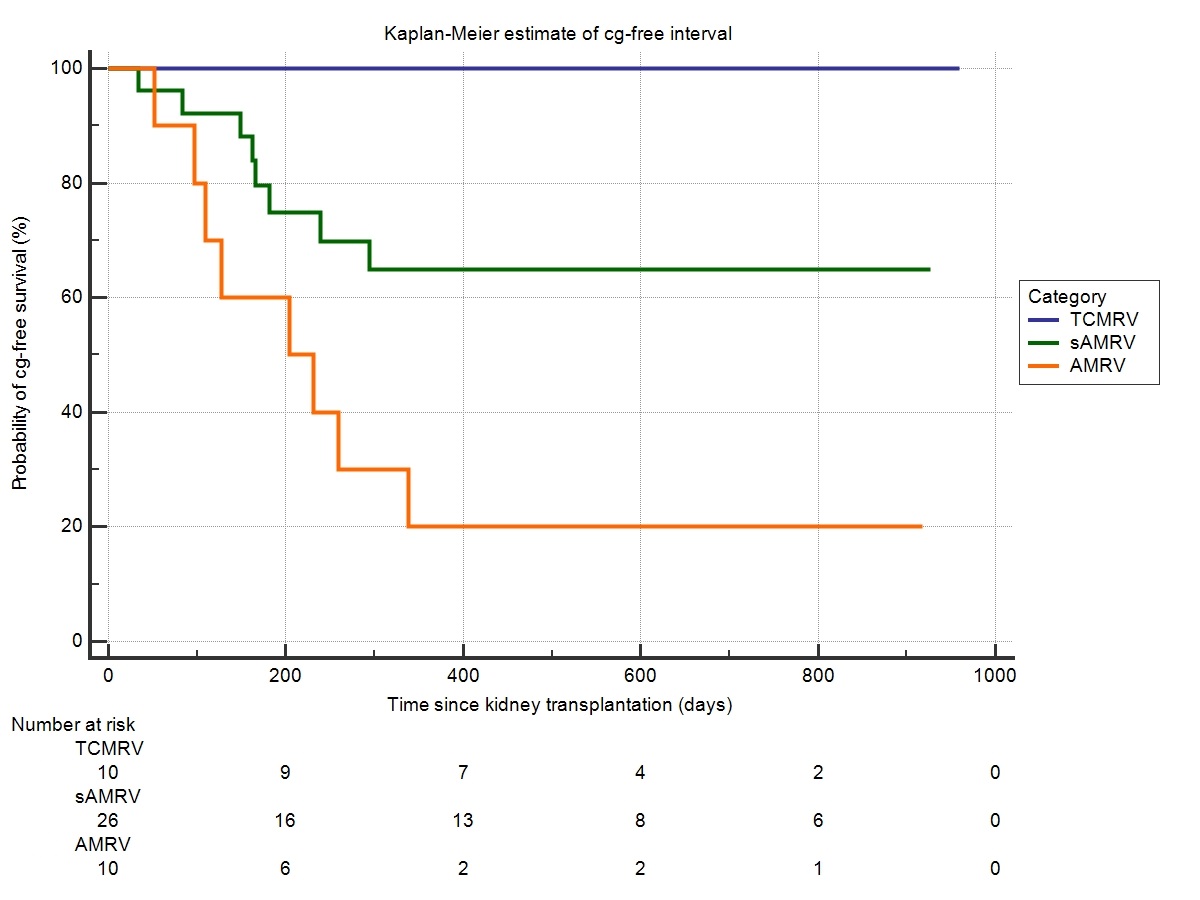 Overall death-censored graft survival (DCGS) was worst in AMRV (60%) compared to sAMRV (92%) and TCMRV (100%) (Figure 2) declaring AMRV phenotype to be associated with the highest risk of graft failure [HR 6,9 (95% CI 1.4-33.7; p=0.017)].
Overall death-censored graft survival (DCGS) was worst in AMRV (60%) compared to sAMRV (92%) and TCMRV (100%) (Figure 2) declaring AMRV phenotype to be associated with the highest risk of graft failure [HR 6,9 (95% CI 1.4-33.7; p=0.017)].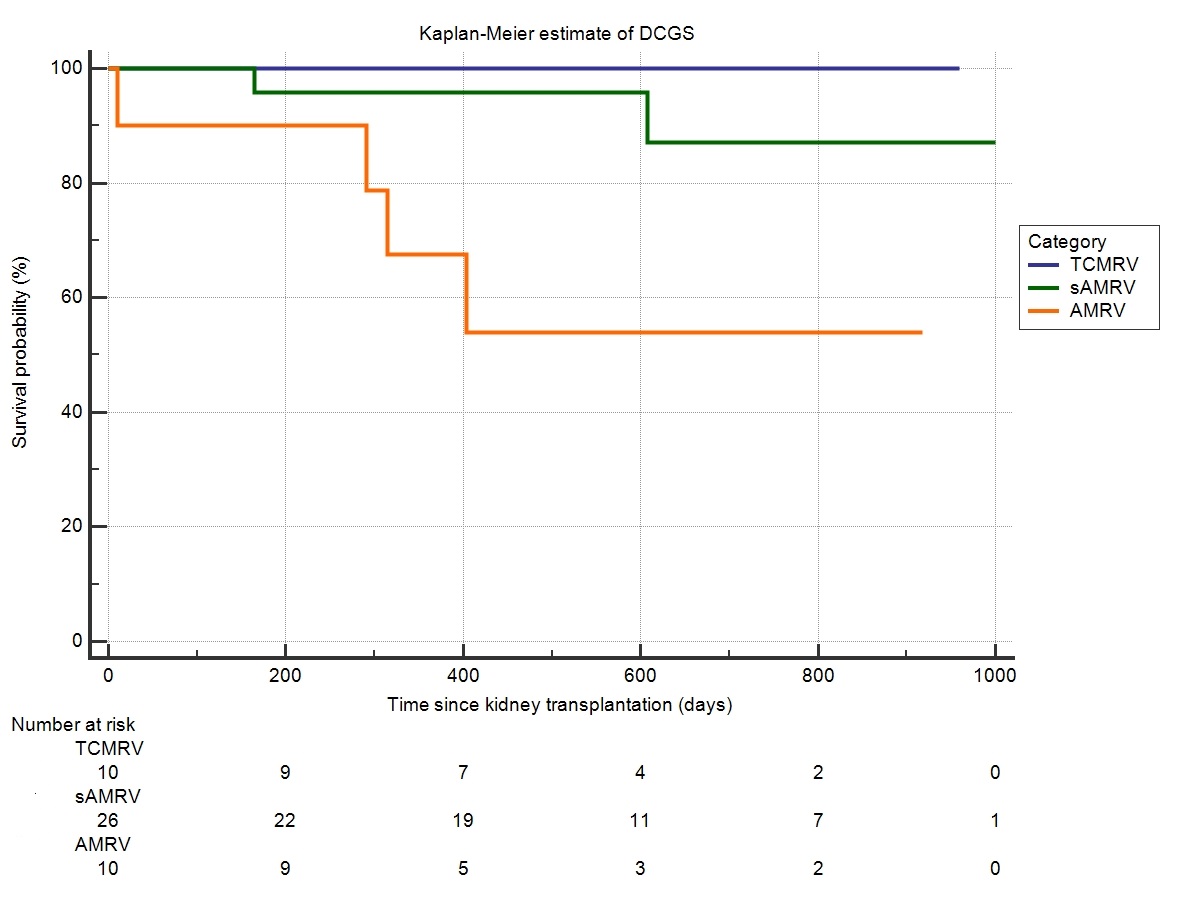
Discussion: AMR is a known major obstacle to long-term kidney graft survival. However, suspected AMRV brings uncertainty into clinical praxis when not completely fulfilling histological Banff definition (Loupy, AJT, 2017). Advanced diagnostic tools like microarray analysis suggest even comparable outcome of DSA and non-DSA (suspected) phenotype of AMR (Garg, Transplant Rev, 2017). Although short term prognosis of sAMRV was quite favourable in our study, findings of transplant glomerulopathy in surveillance biopsies 3 months after diagnosis indicate the risk of premature graft loss (Lesage, Transplantation, 2015). We believe that performing of prospective surveillance biopsies in patients with vascular rejection even under subclinical conditions might be a valid surrogate endpoint for long-term graft prognosis.
Conclusion: Our study confirmed detrimental impact of antibody mediated vascular rejection on one year graft survival. Moreover, suspected AMRV defined as v-lesion associated with microvascular inflammation in the absence of donor specific antibodies might result into early chronic rejection with negative impact on long-term graft survival.
Supported by Ministry of Health, Czech Republic, grant nr. 15-26519A, 17-29992A.
