An Increase in Low-Grade Spot Urine Protein Excretion During the First Year after Kidney Transplantation Associates with Subclinical De-Novo Donor-Specific Antibodies and Microvascular Inflammation at 1-Year Surveillance Biopsies
Miha Arnol1,2, Gregor Mlinšek1, Manca Oblak1, Aljoša Kandus1, Nika Kojc3, Blanka Vidan-Jeras4, Jadranka Buturović-Ponikvar1,2.
1Department of Nephrology, University Medical Centre Ljubljana, Ljubljana, Slovenia; 2Department of Internal Medicine, Medical Faculty, University of Ljubljana, Ljubljana, Slovenia; 3Institute of Pathology, Medical Faculty, University of Ljubljana, Ljubljana, Slovenia; 4Tissue Typing Centre, Blood Transfusion Centre of Slovenia, Ljubljana, Slovenia
Introduction. The presence of microvascular inflammation (MVI) in kidney transplant biopsies often relates to occurrence of donor-specific antibodies (DSA) and antibody-mediated rejection. Here we investigated whether an increase in spot urine protein excretion during the first year after kidney transplantation associates with subclinical de-novo DSA and different Banff MVI scores at 1-year surveillance biopsies.
Patients and Methods. This prospective observational study included 79 consecutive non-sensitized patients who received a deceased donor kidney transplant between Dec 2013 and Jan 2016. All patients received basiliximab induction and tacrolimus-based immunosuppression. Estimated protein excretion rate (ePER) was calculated monthly from spot urine protein-to-creatinine ratios. At 1 year, all recipients underwent surveillance graft biopsy and were screened for de-novo DSA. Screening-positive sera were subjected to single antigen bead (SAB) testing (LABScreen SAB assays; One Lambda). The presence of subclinical de-novo DSA was determined on the basis of SAB reactivity patterns using a mean fluorescence intensity threshold >1000 and stable graft function within the first year (variability in serum creatinine <25% from baseline). Biopsies were read by a single pathologist and presence of MVI (any glomerulitis [g] and/or peritubular capillaritis [ptc] score >0) was graded in accordance with Banff 2013 criteria.
Results. Among the 79 study patients, 10 (13%) developed de-novo DSA at 1 year after transplant (5 patients class I, 5 patients class II). At 1-year surveillance biopsy, 12 patients (15%) had histologic evidence of MVI with g and/or ptc score >0. Among them, 7 patients had mild MVI with [g+ptc] <2, and 5 patients had moderate MVI with [g+ptc] ≥2 and g ≥1. There was no evidence of transplant glomerulopathy or peritubular capillary basement membrane multilayering in electron microscopy. At 1 year, patients with de-novo DSA had significantly greater mean ePER than those without DSA (870, 95% CI 770–1040 mg/day/1.73m2 versus 192, 95% CI 178–206 mg/day/1.73m2; P<0.001). Compared with absence of MVI, mild and moderate MVI were associated with a progressive increase in mean ePER at 1 year (no MVI: 190, 95% CI 178–202 mg/day/1.73m2 versus mild MVI: 740, 95% CI 578–926 mg/day/1.73m2 versus moderate MVI: 1022, 95% CI 884–1226 mg/day/1.73m2; P<0.001). Linear mixed effect regression analyses demonstrated a progressive increase and a significant difference in slope of ePER during the first year between patients with no DSA and de-novo DSA at 1 year (P<0.001), and between patients with no MVI, mild MVI, and moderate MVI at 1-year surveillance biopsies (P<0.001) [Figure].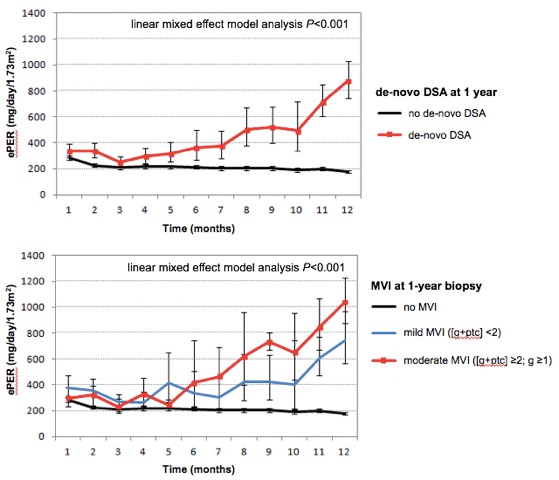
Conclusions. An increase in low-grade ePER during the first year after kidney transplantation associates with subclinical de-novo DSA and the degree of MVI at 1-year surveillance biopsies and may be used as a noninvasive clinical biomarker of endothelial cell injury.
The study was financially supported by the research project of the Slovenian Research Agency (Project ID L3-7582).
