APRIL Serum Levels Relate to Recurrence of IgA Nephropathy
Luis Martin-Penagos1, Adalberto Benito-Hernández1, Javier Martín2, Javier Gómez-Román2, David San Segundo3, Marcos López-Hoyos3, Gema Fernandez-Fresnedo1, Juan Carlos Ruiz1, Emilio Rodrigo1.
1Nephrology, University Hospital Marqués de Valdecilla-IDIVAL-REDINREN, Santander, Spain; 2Anathological anatomy, University Hospital Marqués de Valdecilla-IDIVAL-REDINREN, Santander, Spain; 3Immunology, University Hospital Marqués de Valdecilla-IDIVAL-REDINREN, Santander, Spain
Introduction: The reported incidence of recurrence of IgA nephropathy (IgAN) is about 30%. Although this recurrence rarely manifests before five years, it contributes to further graft loss. Recently, a pathogenic role of some serum biomarkers such as galactose deficient-IgA1, IgG autoantibodies, and IgA–sCD89 complexes has been demonstrated in IGAN recurrence. Although it is known that soluble APRIL regulates production of IgA, induces IgA1 aberrant glycosylation and influences on IgAN development, its role in IgAN recurrence has not been previously analyzed.
Materials and Methods: We selected 33 patients with biopsy-proven IgAN who received a kidney transplant in our hospital. Serum samples were collected and stored at each outpatient visit as routine clinical practice in our centre. Serum soluble APRIL levels were measured by ELISA in sera pre-transplant and at 6 months and 1 and 3 years.
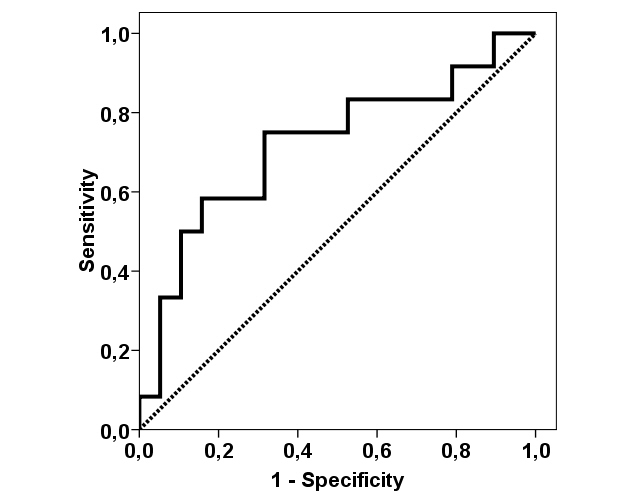
Results and Discussion: Mean follow-up time was 8.4 ± 5.0 years. IgAN recurred in 12 (36%) patients with a mean time to recurrence of 6.2 ± 6.1 years. APRIL value increased significantly from pre-transplant to 1-year only in patients with further recurrence (15.6 ± 17.4 pg/ml vs. 38.2 ± 22.4 pg/ml, p = 0.015), but not in non-recurrent patients (16.4 ± 11.3 pg/ml vs. 26.7 ± 23.1 pg/ml, p = 0.084). Changes in APRIL value did not relate to renal function. Although pre-transplant APRIL values were not significantly different between recurrent and non-recurrent patients (15.6 ± 15.7 pg/ml vs. 16.8 ± 10.8 pg/ml, p = 0.611), mean post-transplant APRIL remained higher in recurrent patients up to 3 years (6-months APRIL 35.9 ± 28.3 pg/ml vs. 18.0 ± 16.6 pg/ml, p = 0.043; 1-year APRIL 40.2 ± 25.7 pg/ml vs. 25.4 ± 21.3 pg/ml, p = 0.096; 3-years 24.9 ± 17.6 vs. 17.9 ± 16.6 pg/ml, p = 0.227). Moreover, 6-months APRIL showed statistically significance for predicting IgAN recurrence (AUC-ROC 0.719, 95%CI 0.524-0.915, p = 0.043).
Conclusion: After kidney transplantation, APRIL serum levels increase more in those patients who will develop IgAN recurrence and this increment remains the following years. We can speculate that APRIL higher levels contributes to IgAN pathogenesis by inducing changes in IgA production and glycosylation, although we did not analyse this issue. Early prediction of IgAN recurrence by measuring APRIL serum levels at 6-months can help to avoid a known risk factor of recurrence such as steroid withdrawal.