Azathioprine Versus Mycophenolate Mofetil in Combination with Tacrolimus and Steroids Maintenance in Pediatric Kidney Transplantation
Sahar Al-Mowaina1.
1Pharmacy, King Fahd Medical City, Riyadh, Saudi Arabia
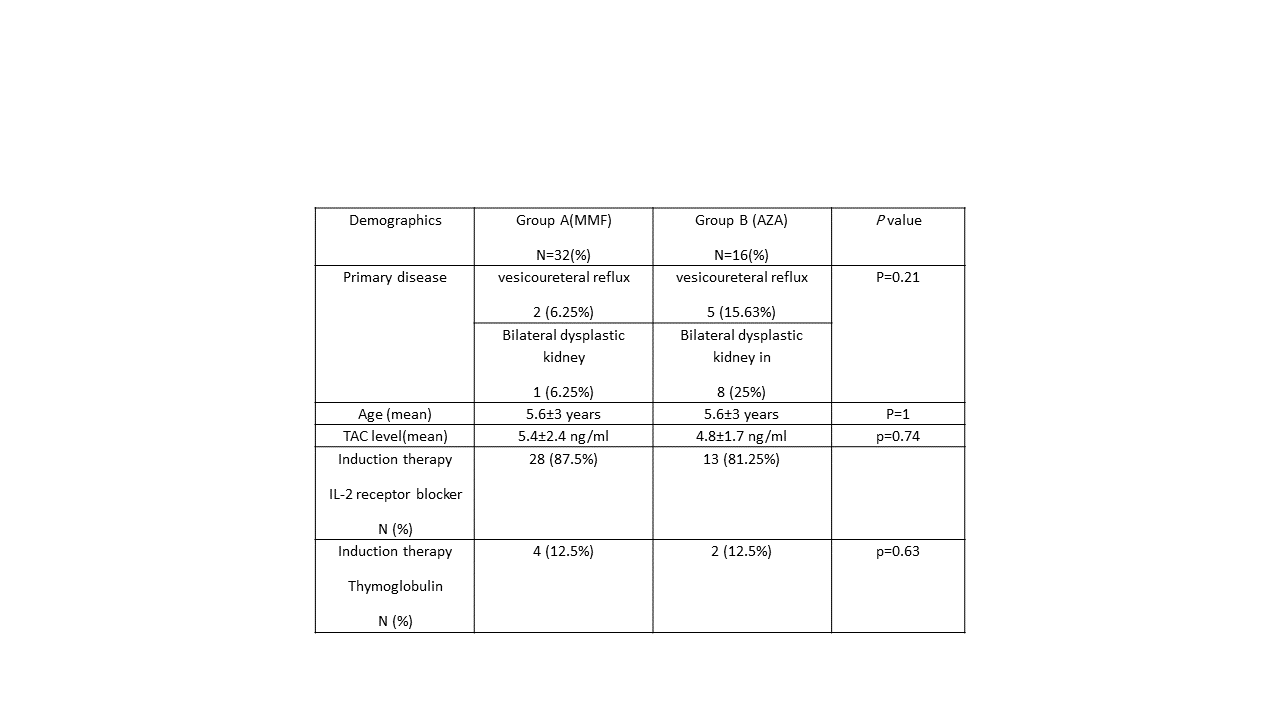 Immunosuppression regimen post kidney transplantation typically consists of triple therapy: calcineurin-inhibitor with tacrolimus (TAC) being the preferred CNI, plus an antimetabolite (mycophenolate mofetil (MMF)or azathioprine(AZA)) and corticosteroids. Limited evidence, if any, supports superiority of MMF in TAC-based protocols in pediatric renal transplant population. At our institution, patients who can't tolerate gastrointestinal side effects of MMF are switched to AZA. We aim to evaluate the efficacy and safety of AZA vs. MMF given with TAC in pediatrics post-kidney transplant Methods: retrospective, single center comparative matching cohort study that included all pediatric kidney transplant recipients who were switched to AZA from January 2001 to December 2016 and compared to those who continued MMF, patients were followed for a minimum of one year at a large tertiary care hospital with major organ transplant center. Pediatric patients (≤14 years) who were switched to AZA within 3 months post kidney transplantation and continued on it for at least 6 months were included. They were matched in a 2:1 ratio based on age and immunological risk. Patients who were highly sensitized, non-kidney transplant recipients, received CSA and/or re-transplanted were excluded. Primary efficacy endpoints are estimated glomerular filtration rate (eGFR) using Schwartz equation and biopsy proven acute rejection (BPAR) both at 1-year post-transplantation. Patient/graft survival at 1-year was considered as a secondary endpoint. Adverse events were assessed as safety endpoints. Data were collected using patients’ charts and electronic medical records. Baseline parametric data, primary and secondary outcomes were expressed as mean ± standard deviation or frequencies and percentages. Continues data analyzed using Student’s paired t-test while categorical variables analyzed using chi-square test. A p-value of <0.05 was considered statistically significant. Statistical analysis conducted using the software package SAS version 9.3. Results: Out of 334 pediatric patients who underwent kidney transplant from 2001 to 2016, 16 patients received AZA and fulfilled our selection criteria were included and matched to 32 patients in MMF group demographics(table1).The mean eGFR after 1-year follow-up was 76.9±39.2 ml/min in AZA group and 89.2±27.2 ml/min in MMF group (P=0.21). Four patients (25%) in AZA group had BPAR compared to five patients (15.6%) in MMF group, (p=0.456). There was no statistically significant difference between groups with regards to adverse events. One patient in each group required dialysis due to loss of graft function. None of the patients died during the study period Conclusion: This is the largest cohort ever performed to evaluate the safety and efficacy of AZA in pediatric renal transplant patients as part of TAC containing regimen. Our study showed no difference in eGFR, BPAR, patient and graft survival between AZA and MMF in one year follow-up period, however, larger prospectively designed studies are needed to confirm this finding.
Immunosuppression regimen post kidney transplantation typically consists of triple therapy: calcineurin-inhibitor with tacrolimus (TAC) being the preferred CNI, plus an antimetabolite (mycophenolate mofetil (MMF)or azathioprine(AZA)) and corticosteroids. Limited evidence, if any, supports superiority of MMF in TAC-based protocols in pediatric renal transplant population. At our institution, patients who can't tolerate gastrointestinal side effects of MMF are switched to AZA. We aim to evaluate the efficacy and safety of AZA vs. MMF given with TAC in pediatrics post-kidney transplant Methods: retrospective, single center comparative matching cohort study that included all pediatric kidney transplant recipients who were switched to AZA from January 2001 to December 2016 and compared to those who continued MMF, patients were followed for a minimum of one year at a large tertiary care hospital with major organ transplant center. Pediatric patients (≤14 years) who were switched to AZA within 3 months post kidney transplantation and continued on it for at least 6 months were included. They were matched in a 2:1 ratio based on age and immunological risk. Patients who were highly sensitized, non-kidney transplant recipients, received CSA and/or re-transplanted were excluded. Primary efficacy endpoints are estimated glomerular filtration rate (eGFR) using Schwartz equation and biopsy proven acute rejection (BPAR) both at 1-year post-transplantation. Patient/graft survival at 1-year was considered as a secondary endpoint. Adverse events were assessed as safety endpoints. Data were collected using patients’ charts and electronic medical records. Baseline parametric data, primary and secondary outcomes were expressed as mean ± standard deviation or frequencies and percentages. Continues data analyzed using Student’s paired t-test while categorical variables analyzed using chi-square test. A p-value of <0.05 was considered statistically significant. Statistical analysis conducted using the software package SAS version 9.3. Results: Out of 334 pediatric patients who underwent kidney transplant from 2001 to 2016, 16 patients received AZA and fulfilled our selection criteria were included and matched to 32 patients in MMF group demographics(table1).The mean eGFR after 1-year follow-up was 76.9±39.2 ml/min in AZA group and 89.2±27.2 ml/min in MMF group (P=0.21). Four patients (25%) in AZA group had BPAR compared to five patients (15.6%) in MMF group, (p=0.456). There was no statistically significant difference between groups with regards to adverse events. One patient in each group required dialysis due to loss of graft function. None of the patients died during the study period Conclusion: This is the largest cohort ever performed to evaluate the safety and efficacy of AZA in pediatric renal transplant patients as part of TAC containing regimen. Our study showed no difference in eGFR, BPAR, patient and graft survival between AZA and MMF in one year follow-up period, however, larger prospectively designed studies are needed to confirm this finding.
