Improvement of Metabolic Function after Intra-Portal Allogeneic Islet Transplantation Using Induction Treatment of the Non-Hematopoetic Erythropoietin Analogue Cibinetide in a Mouse Model
Ming Han Yao1, Masaaki Waatanabe2, Sune Sun1, Kazuaki Tokodai1, Anthony Cerami3, Michael Brines3, Bo Göran Ericzon1, Torbjörn Lundgren1, Makiko Kumagai-Braesch1.
1Department of Transplantation surgery, Karolinska University Hospital , Stockholm, Sweden; 2Department of Gastroenterological Surgery I, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 3Araim Pharmaceuticals, Tarrytown, NY, United States
Introduction: Preventing early immune reactions immediately after PITx is increasing recognized for controlling progressive allograft rejection. Recently, we have demonstrated that cibinetide, a non-hematopoetic erytropoetin analogue, protected pancreatic islets and ameliorated the inflammatory response following PITx leading to better islet engraftment. In this study, we investigate the effect of cibinetide on adaptive immunity with focuses on its efficacy as induction treatment in allogenic PITx and dendritic cell development.
Material and Methods: Pancreatic islets isolated from BALB/c mice were transplanted into the recipient livers of streptozotocin induced diabetic B6 mice via the portal vein. In the first set of the study, recipient animals were received 330-450 islets andwere treated with (1)cibinetide (120 µg/kg) intraperitoneally preoperatively on the transplantation day (just before and at 0, 6 hrs after PITx), and once daily thereafter until day 14 days after. (2)Vehicle (PBS) was administered to the control group.
In the second set of the study, mice, received 450 islets and treated with: (3)Low low dose tacrolimus (0.4 mg/kg) i.p daily from day4 until day14 or (4)combination treatment of cibinetide (120 µg/kg) perioperatively and once daily for 14 days and tacrolimus (0.4 mg/kg) from day4 to day14. Non-fasting blood glucose levels were monitored daily as indication of graft function. Livers were harvested at euthanasia and histological examination was performed.
Mouse bone marrow derived dendritic cells (DC) were differentiated with mouse GM-CSF and IL-4 with or without cibinetide (100 nM). LPS (100 ng/ml) was added at day6 of the culture for DC maturation. DC maturation was compared in-vitro.
Result and Discussion: Treatment with cibinetide significantly prolonged graft survival compared to PBS treated control group, however, most of the grafts gradually rejected during the treatment period.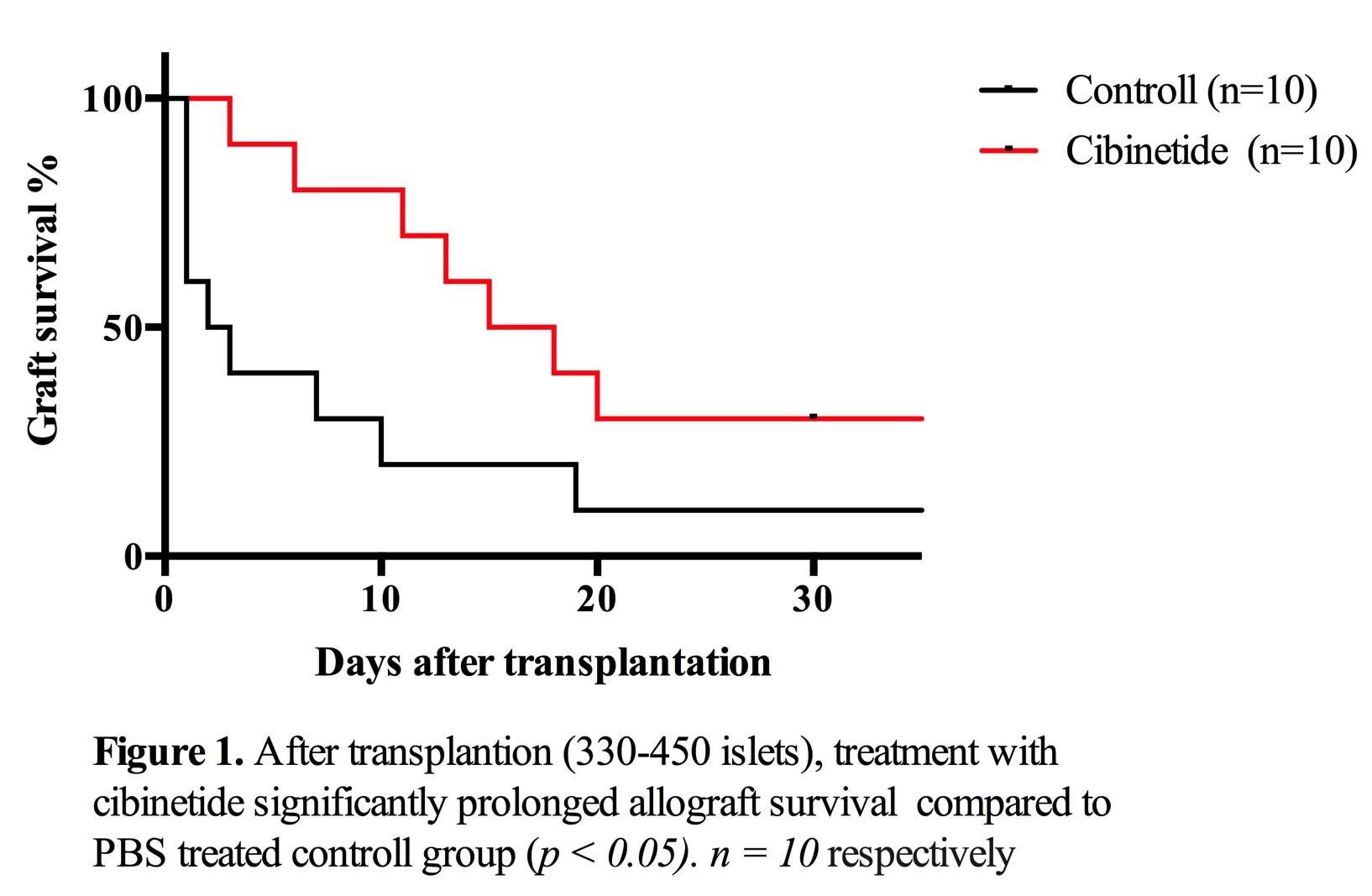 At five days after transplantation, transplanted islets could not be detected in the vehicle treated group, whereas several insulin positive regions with severe infiltration of CD11b+ cells in the liver was detected in cibinetide treated group.
At five days after transplantation, transplanted islets could not be detected in the vehicle treated group, whereas several insulin positive regions with severe infiltration of CD11b+ cells in the liver was detected in cibinetide treated group.
The tacrolimus treated mice had an MST of 28,5 days, while in the combination treatment group, 4/5 mice maintained euglycemic at day 50.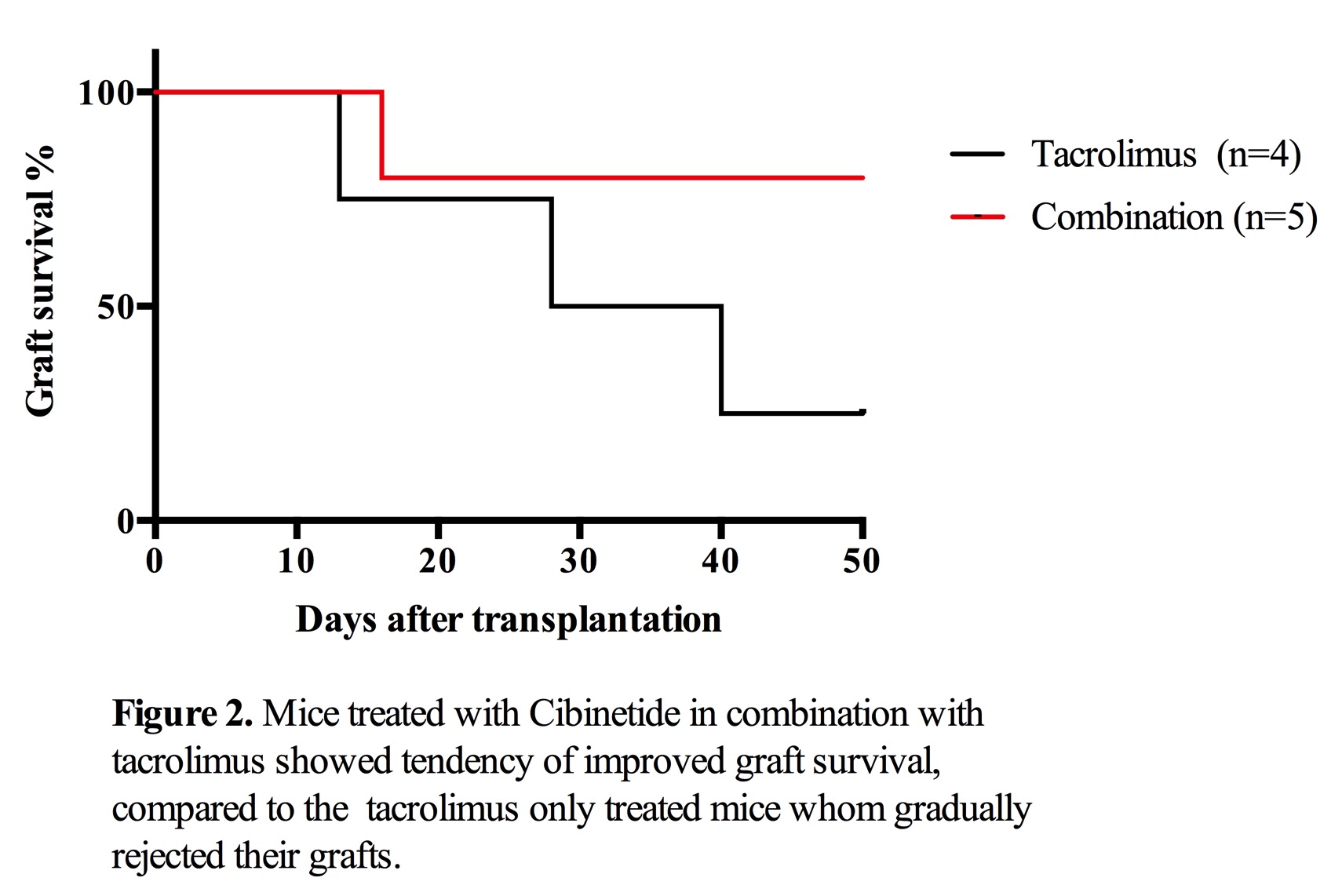 Insulin positive area were found in the livers of combination treatment group, which maintained euglycemia more than 50 days. No lymphocyte infiltration was detected.
Insulin positive area were found in the livers of combination treatment group, which maintained euglycemia more than 50 days. No lymphocyte infiltration was detected.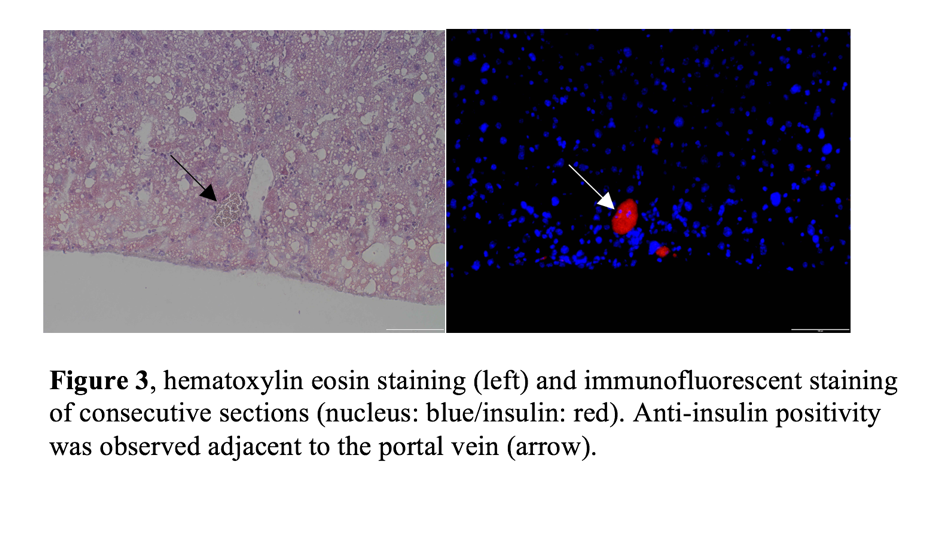
Cibinetide treatment reduces DC maturation in-vitro, shown by lower expression of MHC ClassII antigens (approximately 10% lower mean fluorescent intensity, p<0.05) and lower production of IL-12 (920±25 vs 750±22 ng/ml, p<0.05).
Conclusion: Combination therapy using cibinetide and low dose tacrolimus could be useful option to prolong islet allograft survival. Cibinetide treatment may reduce DC activation, which can delay the onset of adaptive immune responses.
