Use of ATG FR in Mode of Single Dose in Recipients of Renal Transplantation. Experience of 2 Transplant Units of Sante Fe, Argentina
Mariano Arriola1,2, Luis E Gaite1, Claudia Favalli1,2, Joaquin Agusti1, Judit Gaite1, Jose Paladini1, Ricardo Ordoñez2, Paulina Risso2.
1Renal Transplant, Clinica de Nefrologia, Santa Fe, Argentina; 2Renal Transplant and Nephrology, Hospital J.M. Cullen , Santa Fe, Argentina
Introduction: The use of ATG FR is common practice as induction in a renal transplant with moderate or severe immune risk. Also receiving patient with receive kidney to expanded criteria’s donor. In this review present of data retrospective, to near safety and efficiency on the use of ATG songle dose in bolus intraoperative.
Objectives: Asses the safety and efficacy of ATG induction in this modality.
Parameters evaluated in efficacy were: GDF, acute rejection (defined as a requirement of dialysis in the first week after transplant). Presence of auto rejection confirmed by biopsy and 09 bannf classification.
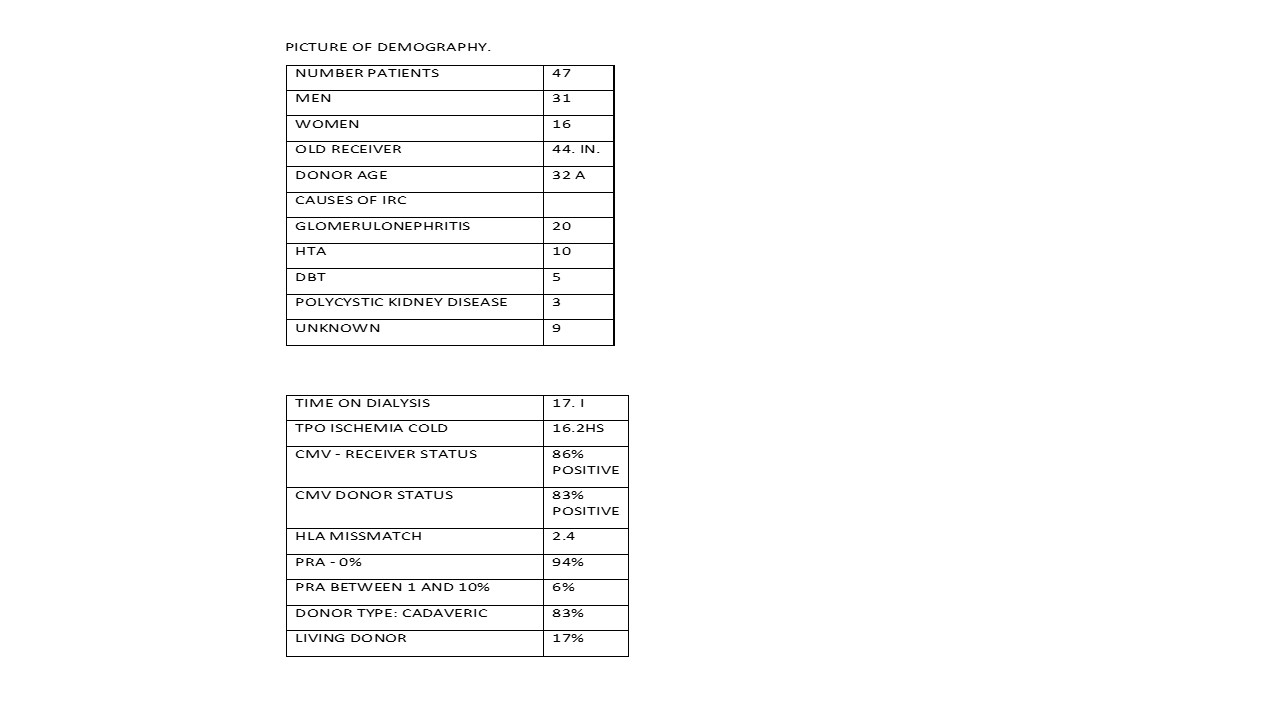
Methods and material: A total of 47 patient to receive cadaveric or live renal transplant, first or second transplant, in a two renal centers of Santa Fe city, between 2013-2016 years. It defined the use of ATG single dose according to immunological characteristics of moderate or high risk with pra greater cero, high mismatch HLA, transplant prior, and other indication that DGF, expanded criteria donor.
Immunosuppression: Single dose intraoperative, 9 mg -kg -dose, diluted in 400 cc saline solution 0.9 % administered slowly ( 8 hours), begin the infusion at anesthesy induction.
The rest of immunosuppression , since DYA cero: advagraf 0.2 mg kg day, mmf or mms a full dose or sirolimus 2 mg day or everolimus at 3 mg day, and metilprednisolona for 0,1 and 2 days, and then meprednisona oral in decrease dose.
Define luekopenia wbc less 3000, plaquetopenia platelets les 75000, . These parameters were measured on day 0-5-15-30-60-90-and 180. Also were measured total peripheral lymphocites.
Be performed universal phrofilaxixs to cmv with ganciclovir or valganciclovir for 3 to 6 month, and also use cotrimoxazol for 3 month.
Picture of demography
Results:
Acute rejection: 14% (7 of 47 patients)
Type rejection: 4 patients: 1a- 2 p. 1b- 1 p, 2a.
Not there was humoral rejection and all were steroids responded.
DGF 17% ( 8 of 47 patients).
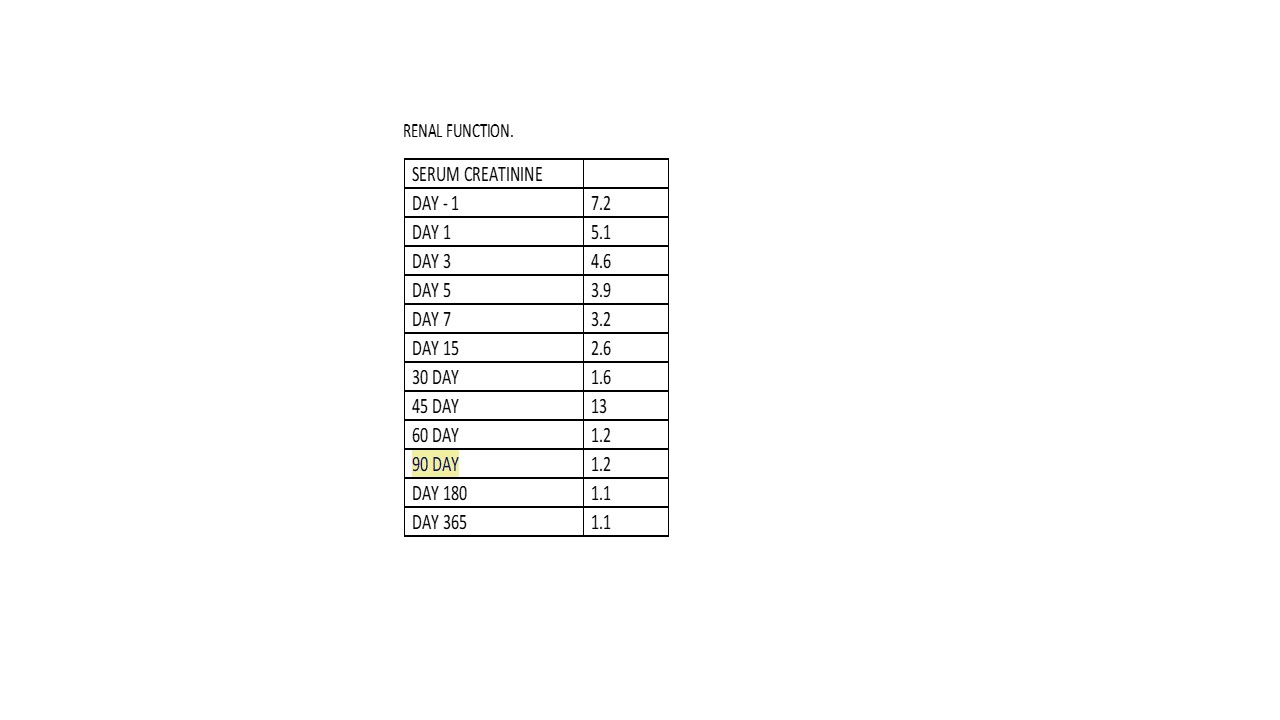
Renal function
Security: Is note down the WBC and platelets between days 1 to 7, and starting the day 8 begin to rise with full recovery at fay 15.
Leukopenia 21%
Thrombocytopenia 26%
We observed the total lymphocytes decreased immediately after ATG infusion, is maintained at low levels until day 6, and is recovered to normal values around day 12-13.
Adverse effects: Fever 4.2% hypotension 6,3 % chills 4.2% leukopenia 21% plaquetopenia 25%.
Infections tabulated within first year: CMV 15% urinary infection 25% pneumonia 21% zoster 12% herpes simplex 24% oral thrush 27% pulmonary histoplasma 2% crain cryptococcus 2% lung and skin nocardia 2%.
Neoplams: Ptld 2%
Skin basal cell 2%
Conclusion: In summary of them data ound in our study we can conclude that the use of single dose ATG FR intraoperative in our population results security and efficacy similar to the scheme suse policlonal antibody.
