Effect of Interferon-Gamma Gene Polymorphism +874 A/T on Cytomegalovirus Infection in Kidney Transplant Recipients with and without Prophylactic Treatment with Valganciclovir
Jose Luis Santiago1, Isabel Pérez-Flores 2, Luis Sánchez-Pérez 1, María Ángeles Moreno de la Higuera 2, Javier Querol-García 1, Natividad Calvo Romero 2, Miguel Fernández-Arquero 1, Esther Culebras 3, Elena Urcelay 1, Cristina Fernández-Pérez 4, Ana Isabel Sánchez-Fructuoso 2.
1Immunology Department, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos (IdISSC), Madrid, Spain; 2Nephrology Department, Hospital Clínico San Carlos, Facultad de Medicina, Universidad Complutense de Madrid, IdISSC, Madrid, Spain; 3Mycrobiology Department, Hospital Clínico San Carlos, Facultad de Medicina, Universidad Complutense de Madrid, IdISSC, Madrid, Spain; 4Clinical Research and Methodology Unit , Hospital Clínico San Carlos, Facultad de Medicina, Universidad Complutense de Madrid, IdISSC, Madrid, Spain
Introduction: The specific mechanism that can explain the linking of Citomegalovirus (CMV) infection with diminished graft function is not totally clear due to there are numerous issues involved. Some cytokines with antiviral proprieties such as interferon gamma (IFN-γ) have been studied. In fact, the SNP +874 A/T of IFN-γ gene, which is linked to their levels, has been associated with increased risk of CMV infection in both lung and kidney transplant recipients. Our purpose was to replicate the association of this SNPin an independent population of kidney transplant patients.
Materials and Methods: A retrospective analysis was performed in 600 consecutive transplanted kidney recipients. The SNP+874 A/T (rs2430561) was genotyped by TaqMan chemistry under conditions recommended by manufacturer and analyzed in a 7900HT Fast Real-Time PCR System (Applied Biosystem, Foster City, CA, USA). CMV viral load was determined by quantitative nucleic acid amplification testing (QNAT).
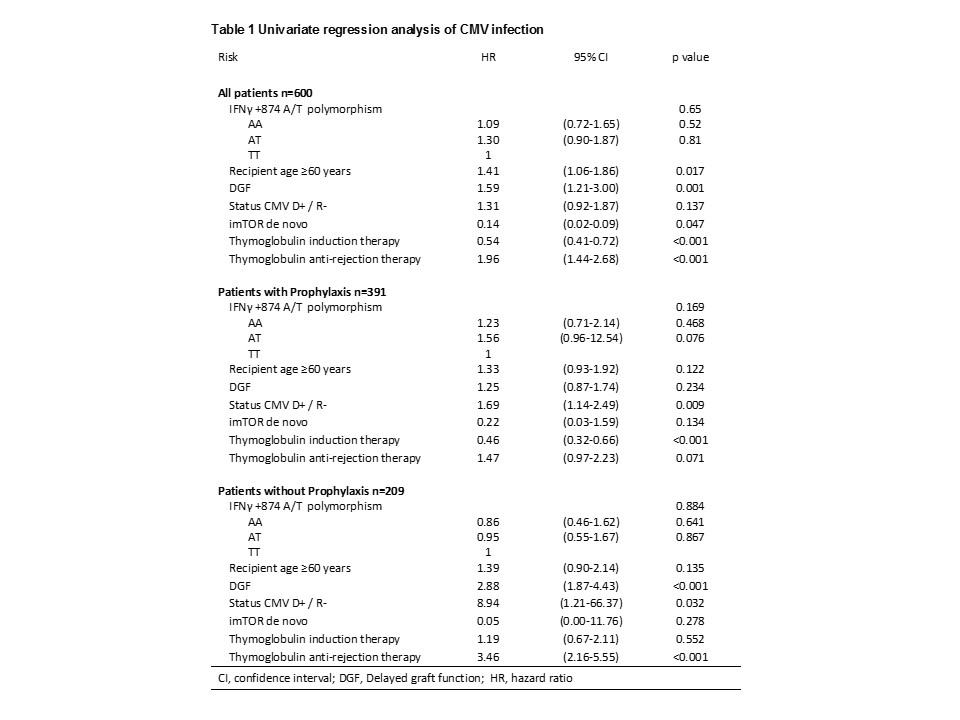
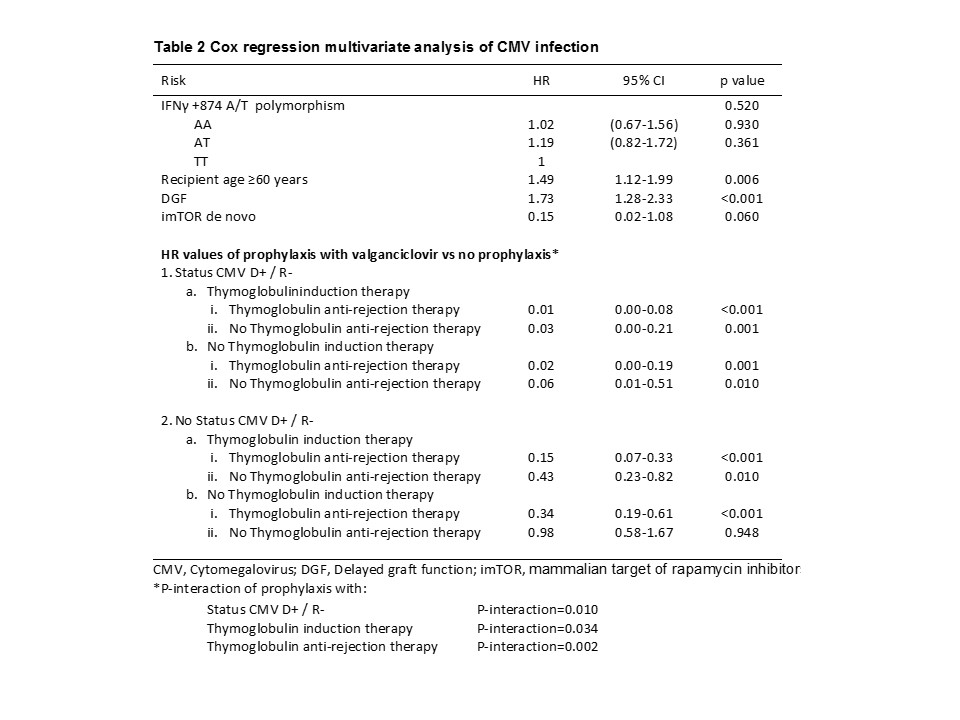
Results and Discussion: Patients with CMV infection were 205 (34.2%) and the median time after transplant to the onset of CMV infection was 2.7 months (range 1.2-5.3 months).We found no association of IFN-γ+874 A/T polymorphism in either univariate or multivariate analyses and regardless of prophylactic treatment with valganciclovir (Tables 1 and 2). Due to antiviral properties of this cytokine and the implication of the aforementioned polymorphism in their level, it would seem the most appropriate approach to assess the risk of CMV infection; however, this association could not be stablished in our well powered cohort. The previously reported association of IFN-γ +874 A/T polymorphism with CMV infection in kidney transplant and lung transplant recipients showed 52 and 42 CMV positives patients respectively. In the adjusted model we found interactions between prophylaxis with status CMV D+ / R- (P-interaction = 0.010), with thymoglobulin induction therapy (P-interaction = 0.034) and with thymoglobulin anti-rejection therapy (P-interaction = 0.002). Data showed that CMV seropositive recipients and no thymoglobulin therapy, the prophylaxis with valganciclovir is not an advantage (Table 2); however if thymoglobulin is used for induction and/or rejection, valganciclovir profilaxis is a protective key for CMV infection (Table 2). Related to well-known associated factors, increased infection risk was observed for recipient age, delayed graft function and (initial immunosuppressive treatment with mammalian target of rapamycin inhibitors (mTORi), (Table 2).
Conclusion: Our data suggest that IFN-γ +874 A/T polymorphism is not a predictive marker of CMV infection in a Spanish cohort of kidney transplant patients, the thymoglobulin therapy is not a risk factor for CMV infection when it is associated with prophylaxis with valganciclovir and the protective effect of imTORis not improved with prophylactic treatment.