Progression of Renal Function after Treatment with the New Antivirals for Hepatitis C: Is There a Difference between Chrinic Kidney Disease and Kidney Transplant Patients?
Ester Casillas Sagrado1, Nuria Maria Rodríguez Mendiola1, Cristina Galeano Alvarez 1, Sara Jiménez Alvaro1, Sandra Elias Triviño1, Ana María Fernández Rodríguez1.
1Nephrology, Ramon y Cajal Hospital, Madrid, Spain
Introduction: Direct acting antivirals (DAA) for the treatment of Hepatitis C have significantly improved the prognosis of this infection, as the rate of patients who achieve sustained virological response is around 98 to 100% in the general population. However, in some cases these antivirals have been associated to an increased progression of chronic kidney disease. We analyze the effects of DAA on the kidney function progression of 25 patients with previously known chronic kidney disease and 14 kidney transplant recipients.
Materials and Methods: We analyzed 39 patients with HCV infection and high viral load who were being followed in our General Nephrology Consult (n = 25 ) and Kidney Transplant Consult ( n = 14 ) . We monitored the progression of kidney function at treatment start, after 3 and 6 months, and 1 year after later. The treatment was prescribed by Gastroenterology and Infectious diseases specialists.
Results: Between May 2014 and February 2016 thirty-nine patients with previously known kidney disease initiated treatment with AAD. 14 patients were kidney transplant recipients with an allograft function ranging from 19 to 76 ml/min at the start of treatment . Among chonic kidney disease (CKD) patients, 19 them were at stage III, 5 at stage IV, and only one patient had stage V CKD. The characteristics are described in table 1.
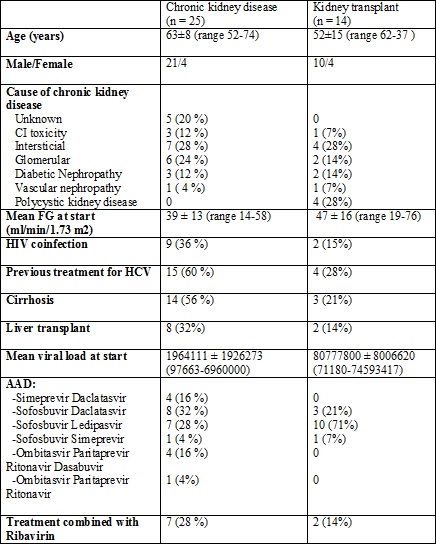
None of the patients presented serious side effects after the start of the treatment. 37 of them presented loss of viral load and sustained virological response (SVR) after 12 weeks and only two of them didn´t respond to the first treatment regimen(Sofosbuvir/Ledipasvir), but obtained SVR after being switched to a different one (Simeprevir/Daclatasvir).
We didn’t observe a significant difference in the kidney function one year after treatment in either group: CKD group: mean GFR at the beginning was 39 ml/ml/1.73 m2. One year later it was 41 ± 16 ml/ml/1.73 m2 (P > 0.05)
Transplant recipients: mean GFR at the beginning was 47,3 ml/ml/1.73 m2. One year later decreased to 45 ± 12 ml/ml/1.73 m2.
We analyzed other parameters, such as hemoglobin and serum albumin but didn’t find significant differences either.
Conclusion: We observed a small improvement of kidney function in the CKD group (2± 12 ml/ml/ 1,73m2). This was not present in the kidney transplant recipients, who suffered a small and non significant decrease of glomerular filtrate rate (-2 ± 9 ml/ml/ 1,73m2) which could be related to prolongued treatment with nephrotoxic drugs such as calcineurin inhibitors.
We can conclude that the AAD are a promising tool in the treatment of HCV infection and its use can be extended to chronic kidney disease and kidney transplant recipients without and accelerated worsening of their kidney function according to our experience. Larger studies are needed to improve the safety of these drugs in these patients.
