ABO Incompatible Renal Transplantation without Anti-CD20 Treatment or Splenectomy: Comparison with Blood Group Compatible Renal Transplantation.
Amit Sharma1, Harinderjit S Gill1, Mukut Minz2, Sunil Kumar2, Apra Kalra3, Divya Singh4, Rupinderjit Kaur1, Monika Rana1.
1Nephrology, Dialysis and Kidney Transplant, Fortis Hospital, Mohali, Punjab, India; 2Transplant Surgery, Fortis Hospital, Mohali, Punjab, India; 3Transfusion Medicine, Fortis Hospital, Mohali, Punjab, India; 4Radiodiagnosis, Prime Diagnostic and Heart Institute, Chandigarh, India
Introduction:The gap between demand and supply of kidneys for patients with end stage renal disease (ESRD) has been decreased by ABO-incompatible (ABOi) renal transplants. The study was conducted to assess the feasibility of performing ABOi renal transplant without Anti CD20 antibody use or splenectomy and compare their outcomes with ABO compatible (ABOc) transplants.
Methods: Study conducted at a tertiary care renal transplantation center included patients for renal transplant from January 2014 to August 2017. A total of 254 patients were included in the study. They were divided into two groups based on ABOi or ABOc donors. Patients received Tacrolimus, Mycophenolate mofetil and Prednisolone, started 10 days prior to tentative date of renal transplantation in case of ABOi and 2 days prior to surgery in case of ABOc transplant. Plasmapheresis and low dose intravenous immunoglobulin (100 mg/kg) was given until an acceptable isoagglutinin titer (1:4) was obtained on the date of transplantation in ABOi cases. All patients received induction immunosuppression: Thymoglobulin (1 mg/kg/day for 3 days) for ABOi and ABOc with HLA mismatch > 3/6 and Basiliximab two doses of 20 mg each in ABOc with HLA mismatch < 3/6 on the day of surgery and post-operative day 4. In ABOi transplant, isoagglutinin titers were regularly monitored and plasmapheresis was done preemptively if they increased to predefined levels (>1:8 in first week post transplant and >1:16 in second & third week post transplant). Main Outcome Measures: Patient and allograft survival; 1-, 3-, 6-, 12-, 24-, and 36-month renal function; infectious complications; and incidence of rejection.
Results: 67.86% of the ABOi recipients and 76.1% of ABOc recipients were males. Mean±Standard Deviation (SD) age of the ABOi recipients was 42.67±11.80 years (41.73±13.68 years in ABOc group). Median isoagglutinin titer at start was 1:32 (1:2 to 1:256). Mean number of plasmapheresis required were 3. In ABOi group, Mean±SD creatinine levels were 1.33±0.30 mg/dL at 1 month, 1.37±0.37 mg/dL at 6 months, 1.33±0.37 mg/dL at 24 months and 1.37±0.35 mg/dL at 36 months. In ABOc group, Mean±SD creatinine levels were 1.20±0.37 mg/dL at 1 month, 1.28±0.48 mg/dL at 6 months, 1.28±0.42 mg/dL at 24 months and 1.29±0.45 mg/dL at 36 months. 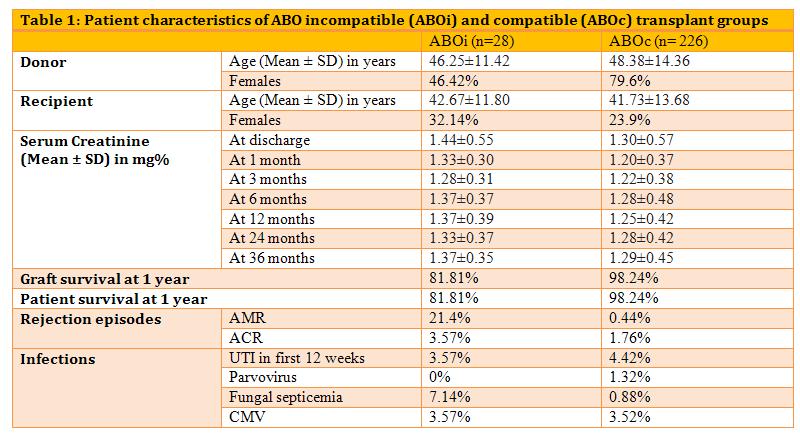
Discussion: There was no significant difference between the post transplant serum creatinine in the two groups. In the ABOi group, there were 6 episodes of biopsy proven acute antibody mediated rejection (AMR) and 1 patient had acute cellular rejection (ACR), treated successfully. One episode of AMR and 4 episodes of ACR were observed in the ABOc group. Two patients succumbed to fungal sepsis in the ABOi group.
Conclusion: This study suggests that successful ABOi renal transplantation is possible without the use of splenectomy or Anti-CD20 treatment but AMR episodes as well as fungal sepsis are significantly high in the ABOi group.
