Sarcolemmal Complement Membrane Attack Complex Deposits During Acute Rejection of Myofibers in Nonhuman Primates
Daniel Skuk1, Jacques P Tremblay1.
1Axe Neurosciences, Research Center of the CHU de Quebec - CHUL, Quebec, QC, Canada
Introduction: Transplantation (Tx) of myogenic cells has potential applications in the treatment of muscle disorders. Excluding purely autologous Tx, the survival of myofibers formed by cell Tx depends on control of acute rejection (AR). Since there were no criteria for diagnosing AR of myofibers in the clinic, we conducted studies in nonhuman primates to fill this void. We already determined the histology of AR in this context. Since preliminary observations showed the presence of complement membrane attack complex (MAC) in the periphery of myofibers during AR, we analyzed whether MAC deposits occurred before, during and after AR of myofibers.
Methods: We allotransplanted muscle precursor cells (MPCs) in macaques immunosuppressed with tacrolimus. To induce AR of allogeneic myofibers produced by the cell Tx, tacrolimus was given for one month (to allow complete myofiber regeneration by the grafted MPCs) and then withdrawn (week 0). MPC-grafted sites were biopsied at tacrolimus withdrawal and then every 2 weeks, being analyzed by histology (hematoxylin & eosin, immunodetection of CD8 and CD4) until the rejection resolution (week 12-16). MAC was revealed by immunodetection of C5b-9. Blood was sampled before Tx, at week 0 and then at every 2 weeks to detect antibodies against donor’s MPCs by flow cytometry.
Results: As described yet, the histological mark of AR is the presence of dense focal accumulations of CD8+ / CD4+ lymphocytes around myofibers. These lymphocyte accumulations appeared in week 4-8, peaked in week 6-14, and then declined to disappear in 4 weeks or more. Circulating antibodies against donor’s MPCs appeared in week 6-10, reached a maximum 2 weeks later, and were elevated for the rest of the follow-up. No MAC was detected in biopsies that preceded the onset of lymphocyte accumulations. At the peak of lymphocyte infiltration, several myofibers exhibited sarcolemmal MAC partially or completely covering the myofiber contour, generally close to lymphocyte accumulations (Figure). Some of these myofibers also had sarcoplasmic MAC labeling (Figure, arrow) indicating membrane damage and necrosis, but the majority had no pathological alterations (Figure, asteriks). Several myofibers with sarcolemmal MAC and no pathological alterations were observed in the biopsies of the following periods until the end of the follow-up, independently of the scarcity or absence of lymphocyte accumulations.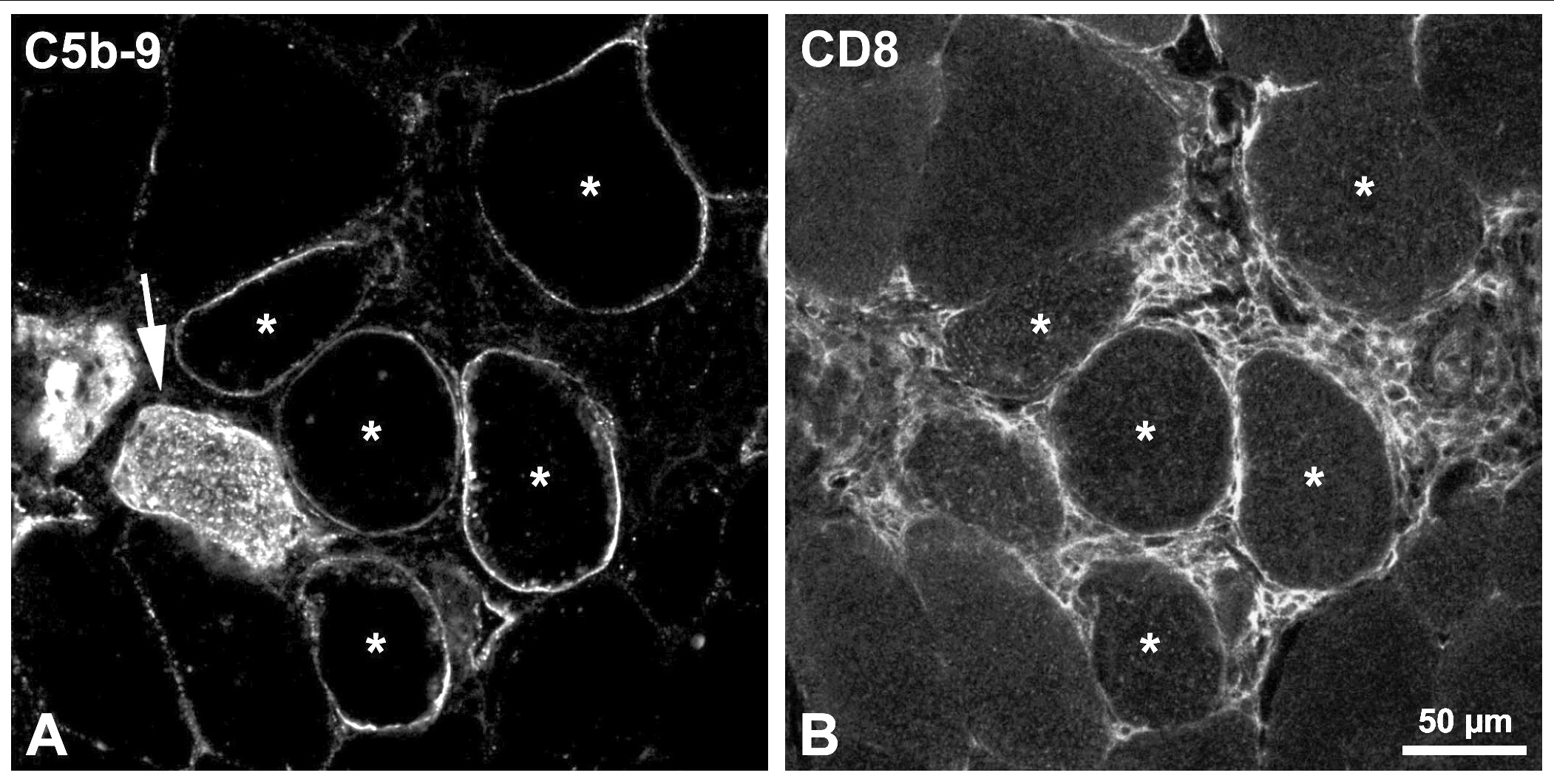
Conclusions: Sarcolemmal MAC deposits appeared concurrently with myofiber AR. Since the vast majority of myofibers with sarcolemmal MAC were histologically normal, we can deduce that MAC is not harmful to myofibers. The persistence of sarcolemmal MAC after the peak of AR is intriguing. From the point of view of biopsy analysis, we suggest that sarcolemmal MAC could be an indicator of present or previous AR. It would be interesting to analyze how long sarcolemmal MAC remains after AR.
Supported by grants of the Jesse’s Journey Foundation for Gene and Cell Therapy of Canada and the Canadian Institute of Health..
