Predicting Early Viral Control under DAA Therapy for Chronic HCV Using Pretreatment Immunological Markers
Akinbami R. Adenugba1, James A. Hutchinson1, Kilian Weigand2, Katharina Kronenberg1, Jan Haarer1, Florian Zeman3, Paloma Riquelme1, Matthias Hornung1, Norbert Ahrens4, Hans J. Schlitt1, Jens M. Werner1, Edward K. Geissler1.
1Department of Surgery, University Hospital Regensburg, Regensburg, Germany; 2Department of Gastroenterology, Endocrinology, Rheumatology and Infectious Diseases, University Hospital Regensburg, Regensburg, Germany; 3Center for Clinical Studies, University Hospital Regensburg, Regensburg, Germany; 4Department of Clinical Chemistry and Laboratory Medicine, University Hospital Regensburg, Regensburg, Germany
Introduction: The introduction of all-oral direct-acting antiviral (DAA) treatment has revolutionized care of patients with chronic hepatitis C virus (HCV) infection. Regrettably, the high cost of DAA treatment is burdensome for healthcare systems and may be prohibitive for some patients who would otherwise benefit. Understanding how patient-related factors influence individual responses to DAA treatment may lead to more efficient prescribing.
Materials and Methods: In this observational study, patients with chronic HCV infection were comprehensively monitored by flow cytometry to identify pre-treatment immunological variables that predicted HCV RNA negativity within 4 weeks of commencing DAA treatment. Patients were classified as “fast responders” if HCV RNA <12 IU/ml by 4 weeks, or “slow responders” if HCV RNA ≥12 IU/ml at 4 weeks, irrespective of the later outcome. Twenty-three patients (genotype 1a (n=10), 1b (n=9) and 3 (n=4) were treated with daclatasvir plus sofosbuvir (n=15), ledipasvir plus sofosbuvir (n=4) or ritonavir-boosted paritaprevir, ombitasvir, and dasabuvir (n=4).
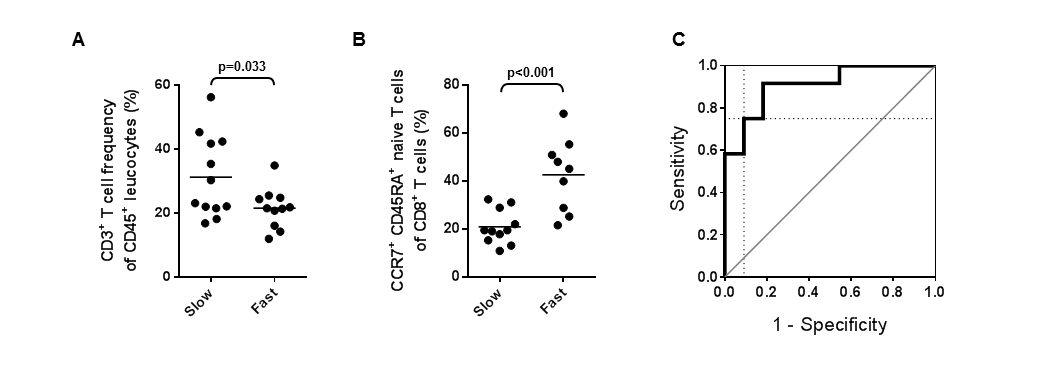
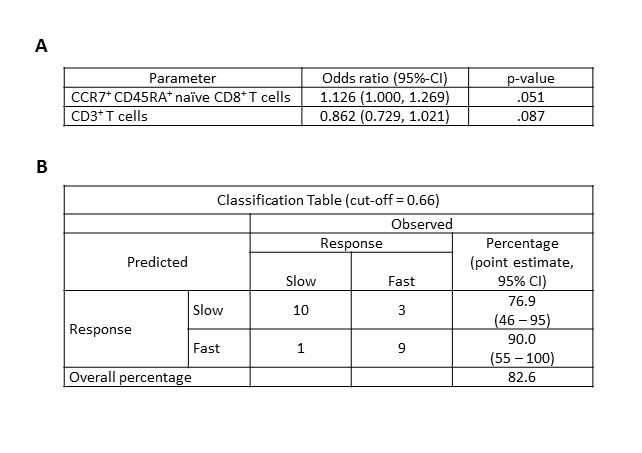
Results and Discussion: Univariate analyses were performed to determine which immunological parameters affected by DAA treatment were most significantly associated with fast or slow responder status. Frequencies of CD3+ T cells with respect to CD45+ leucocytes (Figure.1A) and CCR7+ CD45RA+ naïve CD8+ T cells with respect to CD8+ T cells (Figure.1B) were then entered into a binary logistic regression model as the two most significant and biologically non-redundant independent variables (Table 1A). Receiver operator characteristic (ROC) curve analysis (Area=0.909; S.E.=0.61) was used to determine a cut-off value of the predicted probabilities that maximized both sensitivity (75.0%) and specificity (91.0%) (Figure.1C). Using a cut-off value of 0.66, pre-treatment frequencies of CD3+ T cells and CCR7+ CD45RA+ naïve CD8+ T cells correctly classified 82.6 % of patients as fast or slow responders (compared to 52.2% with no model, i.e. classifying all patients as fast responders) in our study cohort (Table 1B). Notably, the 95% confidence intervals (CI) of the Odds Ratios were narrow and the model was a good fit (Nagelkerke R-squared = 0.623). In a prospective cohort, these same parameters correctly classified 90% of patients with an F-score of 0.67, which indicates reasonable test accuracy. Slow responders exhibited higher frequencies of CD3+ T cells, CD8+ TEM cells, and CD5high CD27- CD57+ CD8+ chronically activated T cells, which may be attributed to bystander hyper-activation of virus-nonspecific CD8+ T cells.
Conclusion: Non-specific, systemic CD8+ T cell activation predicted a longer time-to-viral clearance. This discovery enables pre-treatment identification of individuals who may not require a full course of DAA therapy; in turn, this could lead to individualized prescribing and more efficient resource allocation, especially in patients listed for liver transplantation.