De Novo or Early Conversion to Everolimus and Cancer Incidence in Kidney Transplant Recipients: A Trial-Based ANZDATA Linkage Study
Tracey Ying1,2, Germaine Wong3,4, Wai Lim5, Graham Russ6, John Kanellis7, Helen Pilmore8, David Goodman9, Paul Trevillian10, Scott Campbell11, Michael Suranyi12, Mathew Mathew13, Randall Faul14, Rosemary Masterson15, Philip O'Connell3,4, Steven Chadban1,2.
1Renal Medicine, Royal Prince Alfred Hospital, Camperdown, Australia; 2Charles Perkins Centre, University of Sydney, Camperdown, Australia; 3Centre for Transplant and Renal Research, Westmead Hospital, Westmead, Australia; 4Centre for Kidney Research, The Children's Hospital at Westmead, Westmead, Australia; 5Sir Charles Gairnder, Perth, Australia; 6Central and Northern Adelaide Renal & Transplantation Service, Adelaide, Australia; 7Monash Medical Centre, Clayton, Australia; 8Auckland City Hospital, Auckland, New Zealand; 9St Vincent's Hospital Melbourne, Fitzroy, Australia; 10John Hunter Hospital, Newcastle, Australia; 11Princess Alexandra Hospital, Woolloongabba , Australia; 12Liverpool Hospital, Liverpool, Australia; 13Launceston General Hospital, Launceston, Australia; 14Royal Melbourne Hospital, Parkville, Australia; 15The Alfred Hospital, Melbourne, Australia
Introduction: Cancer after kidney transplantation (KT) is a significant cause of morbidity and mortality. Choice of immunosuppressive regimen may modify the risk of cancer. Mammalian target of rapamycin inhibitors have been shown to have anti-oncogenic effects in both animal and human studies. A large individual patient data (IPD) meta-analysis showed that sirolimus was associated with a significant reduction in the risk of cancer, but at the cost of an increased risk of death. We aimed to determine the cumulative incidence of cancer in KT recipients randomised to de novo or early switch to everolimus (EVL) maintenance immunosuppression versus controls on standard calcineurin inhibitor (CNI) based triple therapy.
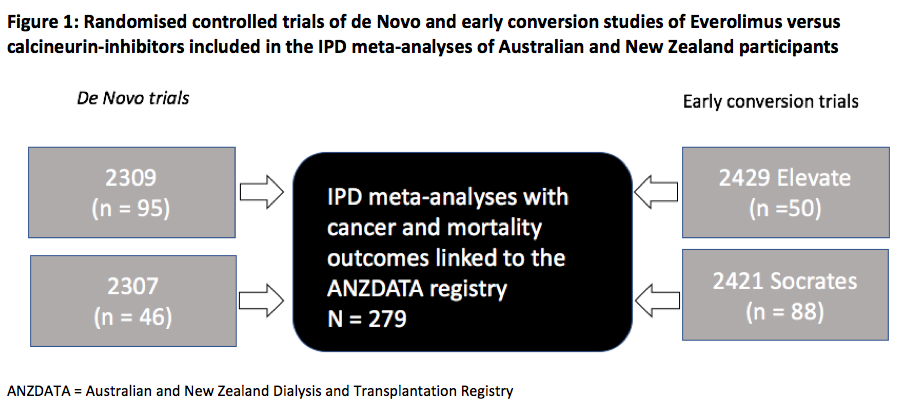
Materials and Methods: We included all de novo or early EVL switch randomised controlled trials (RCTs) conducted in Australia and New Zealand KT recipients between 2001 - 2012 in the IPD meta-analysis (Fig 1). Participants were linked to the Australian and New Zealand Dialysis and Transplantation Registry (ANZDATA) to capture outcomes of incident cancer and all-cause mortality. We compared treatment allocation to EVL or control, with time to first cancer occurrence using Kaplan-Meier estimates. An adjusted Cox proportional hazards model with random effects using pooled individual patient data from all trials was used to determine the overall risks of cancer and death.
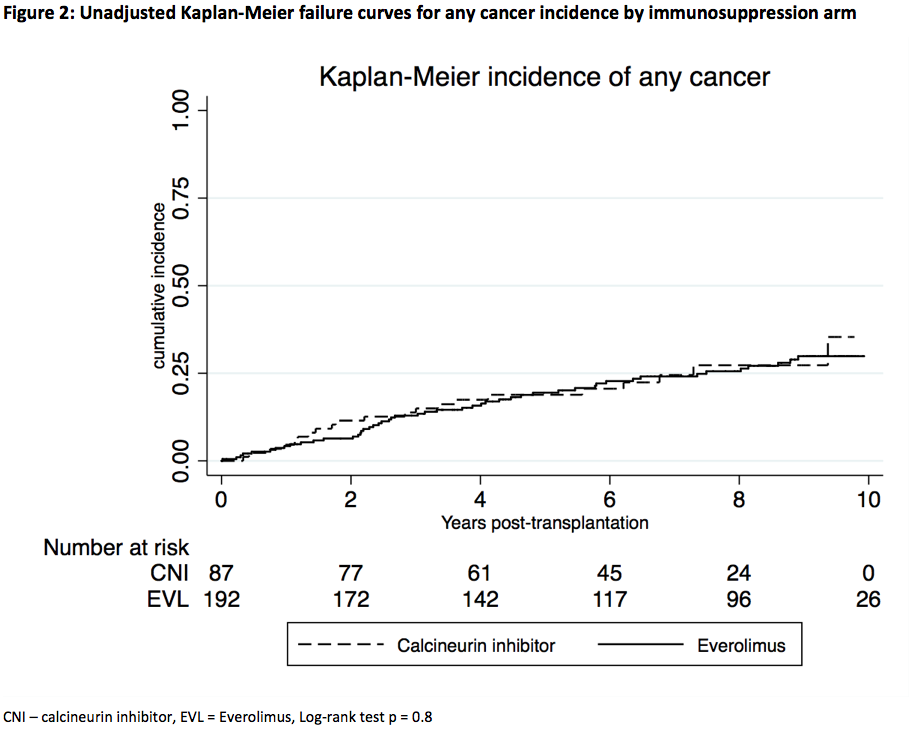
Results and Discussion: The 279 Australian and New Zealand participants (EVL = 192, control = 87) were followed for a median of 9.0 yrs (IQR 6.8, 9.6). Four RCTs examined EVL immunosuppression using de Novo (n=141) or early (<4 mths) (n=138) strategies in addition to either reduced dose CNI or CNI-withdrawal. The control arm consisted of cyclosporine or tacrolimus with mycophenolic acid or azathioprine and steroid maintenance therapy. The cumulative incidence of any cancer was not significantly different between EVL and control (log-rank test p = 0.8) (Fig 2). 30 (15.6%) EVL patients developed incident NMSCs (16 SCCs, 14 BCCs) compared with 17(19.5%) control patients NMSC (8 SCCs, 9 BCCs) (p = 0.7). After adjusting for age and race, the HR for developing any cancer was 0.97 (95% CI 0.58–1.62, p = 0.9) in the EVL group. There was no association between EVL and all-cause mortality (unadjusted HR = 1.20, 95% CI 0.56–2.59). The rate of discontinuation of study drug at years 1 and 2 post-KT was higher in the EVL group compared with controls (year 1 = 29.5% vs 4.5%, year 2 = 41% vs 10.5%, p < 0.001 for both).
Conclusion: In this long-term registry follow-up of 279 trial participants, de novo and early conversion to EVL was not associated with a reduction in the risk cancer compared with CNI-based standard of care. The high discontinuation rate of EVL at 1 and 2 years after transplantation may have contributed to these findings.