Pre-Transplant Immune Markers of Rejection Risk in HIV-Positive Transplant Recipients
Simon Chu1,2, Steven A Wisel3, Brian Shaw4, Karim Lee3, Michelle A Mintz1, Casey Ward3, Emmeline Chuu3, Kristin Melli3, Kentaro Sugisaki3, Peter G Stock3, Qizhi Tang3.
1School of Medicine, University of California, San Francisco, San Francisco, CA, United States; 2Howard Hughes Medical Institute, University of California, San Francisco, San Francisco, CA, United States; 3Department of Surgery, University of California, San Francisco, San Francisco, CA, United States; 4Department of Surgery, Duke University School of Medicine, Durham, NC, United States
Introduction: HIV(+) solid-organ transplant recipients are predisposed to a three times higher rate of rejection episodes when compared to HIV(-) recipients, but immunological correlates of rejection in this population have not previously been identified. Here we describe our investigation of immunologic phenotype and gene expression profiling to identify functional differences between Rejectors (Rej) and Non-Rejectors (NR).
Methods: Donor and recipient peripheral blood mononuclear cells (PBMCs) were collected prior to transplant. Rej were selected based on biopsy-proven acute cellular rejection. Kidney transplant recipients were stratified by Rej (n=28) versus NR (n=56), as compared to matched HIV(-) kidney transplant recipients, HIV(+) non-transplant controls and HIV(-), ESRD(-) healthy control subjects (n=25 per group). These patients were profiled using flow cytometric panels to characterize cellular subsets, activation status, and Treg phenotype. Groups were compared for variance using the Kruskal-Wallis test, with pairwise comparison performed between groups by Dunn’s post-test. For gene expression analysis, pre-transplant HIV(+) liver recipient PBMCs from Rej (n=2) and NR (n=2) were co-cultured in mixed lymphocyte reaction (MLR) in vitro with either CD40L-stimulated donor or 3rd party B cells. Donor B cells were removed by immunodepletion and recipient cells were analyzed using a custom NanoString panel. Raw counts were normalized and p-values were adjusted using the Benjamini-Hochberg procedure.
Results: HIV(+) Rej were found to have markers of increased pre-transplant immune activation as compared to NR, with a bias toward activation of the innate immune system. They exhibited a significantly altered monocyte phenotype, including decreased HLA-DR expression on CD14+CD16+ intermediate monocytes (p<0.0319) [Figure 1A]. Moreover, Rej have increased B cell activation by HLA-DR expression (p<0.0095) [Figure 1B] and less activated Tregs by decreased percentage of CD39+ Tregs (p<0.057) [Figure 1C]. The frequency of Tregs did not differ between the two groups. After alloantigen stimulation, Rej showed increased gene expression of T-cell activation markers, CD28 and ICOS (p=0.0242, 0.0334). Interestingly, NR displayed upregulation of regulatory ligands in the leukocyte immunoglobulin-like receptor (LILR) family, including LILRB1, LILRA1, LILRB4 (p=0.017, 0.0317, 0.0384) [Figure 2B]. Differential gene expression between Rej and NR was preserved irrespective of stimulus by either donor or 3rd party. 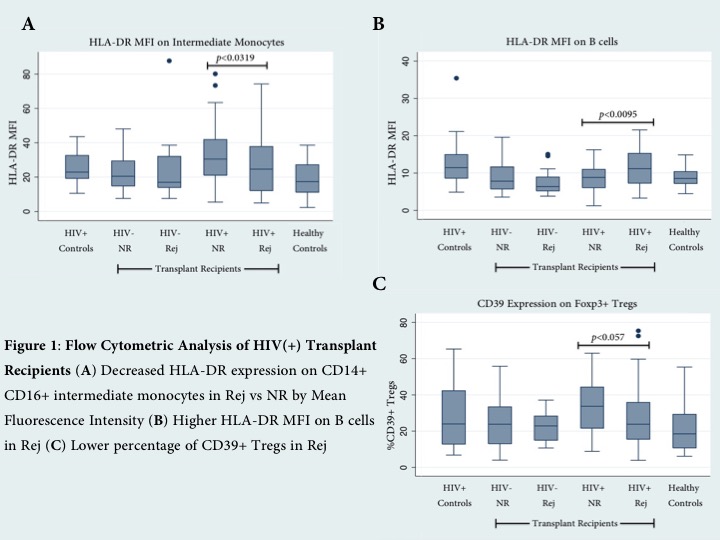
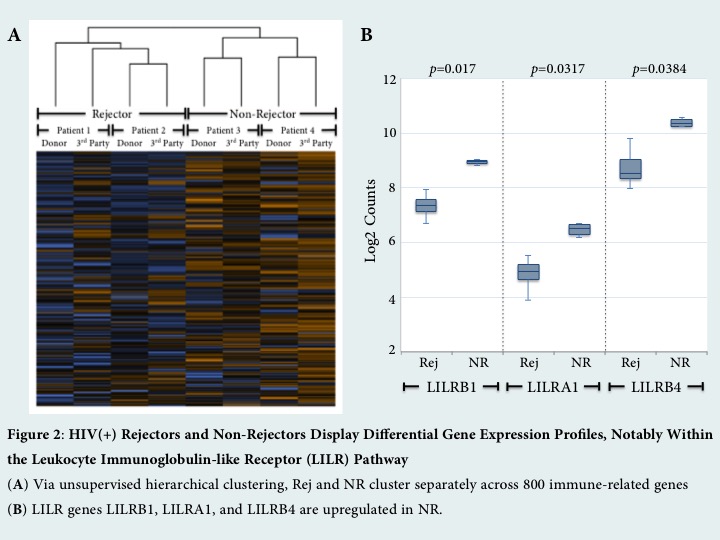
Conclusions: Overall, our results suggest that increased rates of rejection in HIV(+) kidney and liver transplants correlate with pre-transplant, recipient-specific immune dysfunction. Concordance in gene expression profile following stimulation with donor or 3rd party suggests that differential gene expression is an intrinsic, recipient-driven propensity to immune activation in Rej and immune regulation in NR.
