Evaluation of Cell-mediated Immune Response by QuantiFERON Monitor® Assay in Kidney Transplant Recipients
Ivan Margeta1, Ivana Mareković2,3, Ana Pešut2, Marina Zelenika1, Marija Dorotić1, Ivana Mrnjec1, Mladen Knotek1,3.
1Department of Nephrology, University Hospital “Merkur”, Zagreb, Croatia; 2Department of Clinical and Molecular Microbiology, University Hospital Centre Zagreb, Zagreb, Croatia; 3School of Medicine, University of Zagre, Zagreb, Croatia
Introduction: All kidney transplant recipients (KTRs) require immunosuppression, the net level of which is difficult to assess. Current practice in assessing immune reactivity is to monitor levels of some immunosuppressive drugs. QuantiFERON Monitor® (QFM) is an in vitro diagnostic test that detects interferon-γ (IFN-γ) release in peripheral blood. Its clinical utility in assessment of the net state of immunosuppression in KTRs has not been well studied. The aim of our study was to evaluate the discriminating value of QFM testing results for infection and rejection in a single-centre cohort of KTRs.
Methods: Ninety adult KTRs from a tertiary transplant centre were recruited for this observational cohort pilot study. They were divided into three groups by indication for undertaking the QFM test: control (n=59), rejection (n=11) and infection (n=20). The inclusion criteria into the infection group were clinical signs, or symptoms of an infection with elevated CRP (> 20mg/L). Recipients in the rejection group had biopsy-proven kidney rejection. The control group consisted of stable KTRs at various times post-transplant. QFM (Qiagen, Hilden, Germany) was performed according to the manufacturer instructions.
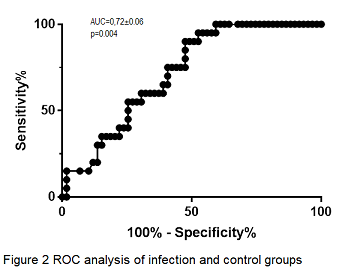
Results Fifty-nine patients were male and 31 female. The mean age was 52.5±1.4 years and mean time after transplantation was 2.6±0.4 years. In the entire cohort age, gender, time after transplantation, serum creatinine concentration, tacrolimus concentration, mycophenolate dose and steroid dose showed no statistically significant association with QFM results. Mean QFM results were 136.24±27.85 IU/mL, 24.84±5.71 IU/mL and 69.44±25.19 IU/mL in the control, infection and rejection group (p=0.04, by analysis of variance), respectively (Figure 1). Post-hoc LSD test revealed a significant difference in QFM results between the control and infection groups (p=0.01, Figure 1), with area under the curve in ROC analysis of 0.72±0.06 (p=0.004, Figure 2). There was no statistically significant difference in QFM results between viral and bacterial infections.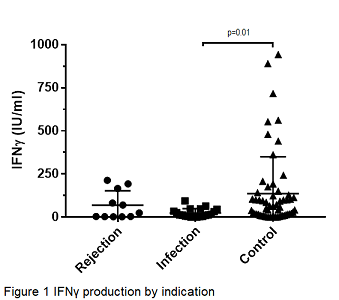
Discussion: A recent study involving pre-transplant liver cirrhosis patients showed lowered QFM values in patients with infective complications, which corroborates our results[1]. However, unlike another study in liver transplant patients, our study did not demonstrate an increase in QFM values in late post-transplant period[2].
Conclusion: QFM results are lower in KTRs presenting with infections. QFM may be used to detect patients at risk for infective complications, but further prospective studies are required.
REFERENCES:
[1] Sood S., Yu L., Visvanathan K., et al. Immune function biomarker QuantiFERON-monitor is associated with infection risk in cirrhotic patients. World J Hepatol. 2016., 1569-1575
[2] Sood S., Cundall D., Yu L., et al. A Novel Biomarker of Immune Function and Initial Experience in a Transplant Population. Transplantation 2014., E50-E51
