Septic Complications and Graft Outcome after Post-Transplant Lymphoproliferative Disorder in Paediatric Solid Organ Transplant Patients in the Rituximab Era
Fang Kuan Chiou1, Sue Beath1, Jane Hartley1, Deirdre Kelly1, Indra van Mourik1, Mona Abdel-Hady1, Patrick McKiernan1, Bruce Morland2, Khalid Sharif1, Girish Gupte1.
1Liver Unit (including small bowel transplantation), Birmingham Children's Hospital, Birmingham, United Kingdom; 2Oncology, Birmingham Children's Hospital, Birmingham, United Kingdom
Introduction: Post-transplant lymphoproliferative disorder (PTLD) is an important cause of morbidity and mortality in children following solid organ transplantation (SOT). Disease remission and survival rates have improved with the introduction of rituximab (RTX) which deletes B-cells, as well as advances in immunologic therapies and chemotherapy regimens. However, treatments for PTLD are associated with significant toxicity and may have additive immunosuppressive effects on the patient.
This study aims to review the septic complications and graft outcomes in children who were treated for PTLD following SOT from 2000–2016 at a single tertiary centre.
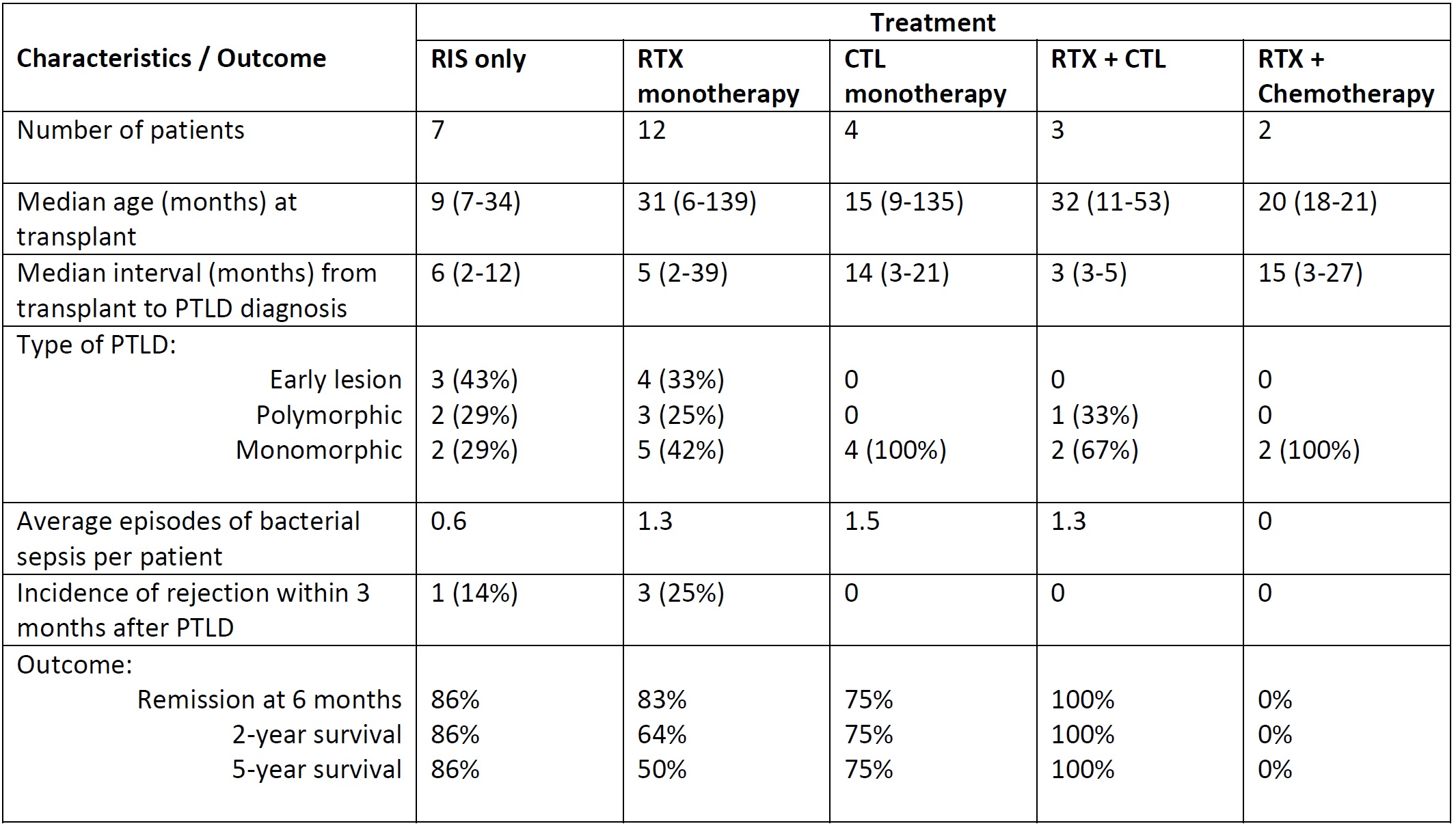
Materials and Methods: Paediatric SOT patients diagnosed with PTLD from 2000–2016 were retrospectively reviewed. PTLD was diagnosed based on World Health Organisation histologic criteria. RTX was introduced to the treatment protocol from the year 2000 and patients received monthly intravenous immunoglobulin (IVIG) infusions for at least 12 months after RTX. Cytotoxic T lymphocyte (CTL) therapy was available in the years 2000–2004 and since 2011. Two, 5 and 10-year data on infective episodes and graft outcome were collected and analysed.
Results and Discussion: Total of 28 patients were included, of whom 15 had isolated liver, 12 had intestinal and 1 had combined liver/kidney transplantation. Median ages at transplant and PTLD diagnosis were 20 months (range: 6 – 139) and 31 months (range: 11 – 178) respectively. Tumours were mostly monomorphic subtype (54%), B-cell origin (96%) and Epstein-Barr virus positive on histology (92%). RTX was given to 17 patients, CTL was given to 7 patients, and 2 patients received chemotherapy. PTLD remission was achieved in 22 patients (79%) while 6 died from PTLD-related cause. Culture-proven bacterial infections occurred in 16 patients (57%) within a median of 3 months (range: 1 – 23) from PTLD diagnosis. Staphylococcus (56%) and Pseudomonas (38%) were the most commonly identified species. Even with IVIG replacement, RTX-treated patients were more likely to have hypogammaglobulinemia (total serum immunoglobulin <4g/L) at 6 months (69% vs 0%, p=0.001) and 2 years (63% vs 0%, p=0.005), recurrent bacterial infections (67% vs 14%, p=0.036) and viral infections (50% vs 0%, p=0.007), most commonly adenovirus, as compared to non-RTX-treated patients. There was no difference in the incidence of rejection, long-term graft hepatitis/fibrosis or re-transplantation between the RTX and non-RTX treated groups. Overall survival rates at 2, 5 and 10 years were 69%, 63% and 60% respectively.
Conclusion: RTX therapy for PTLD, although effective, is associated with significantly higher rates of bacterial and viral infections. Strategies which augment rather than impair the immune system such as CTL, combined with high vigilance for Staphylococcus and Pseudomonas infections, may minimise the morbidity and mortality associated with septic complications in paediatric PTLD survivors.