The Importance of Adjustment for Confounding Factors in Biomarker Studies to Avoid Misleading Conclusions
Sofia Christakoudi1,2, Manohursingh Runglall 1,4, Maria Hernandez-Fuentes1,3.
1MRC Centre for Transplantation, King's College London, London, United Kingdom; 2Biostatistics and Health Informatics Department, Institute of Psychiatry Psychology and Neuroscience, King's College London, London, United Kingdom; 3Global Exploratory Development, UCB Celltech, Slough, United Kingdom; 4NIHR Comprehensive Biomedical Research Centre , London, United Kingdom
GAMBIT Study Consortium.
A number of groups in EU and the USA have been studying Biomarkers of Tolerance in kidney transplant recipients (KTR). Several gene-expression signatures have now been published and validated in different cohornts. However, an important point to consider in stable KTRs selected as tolerant on the basis of a drug-elicited immunological status is whether these patients would retain such features of tolerance once the drugs have been withdrawn.
A fundamental statistical problem complicates the development of biomarkers of tolerance in KTRs. Due to significant ethical constrains for withdrawing IS, tolerance models are trained to discriminate between already established untreated tolerant patients and treated stable KTRs, some of which would be nontolerant and others potentially tolerant. Therefore, two sets of major features discriminate tolerant from non-tolerant KTRs: pharmacological immunosuppression and tolerance.
To illustrate the importance of statistical correction for confounding. We examined the effect of IS drugs on the expression of IGKV4.1 and the effect of IS-drug adjustment on the classification of 175 treated stable KTRs from our GAMBIT dataset (Figure-1). We used as a cut-off for tolerance the median expression of IGKV4.1 among 12 untreated tolerant patients in that series. Our patients received at least one of, prednisolone, calcineurin inhibitor or an anti-proliferative drug. 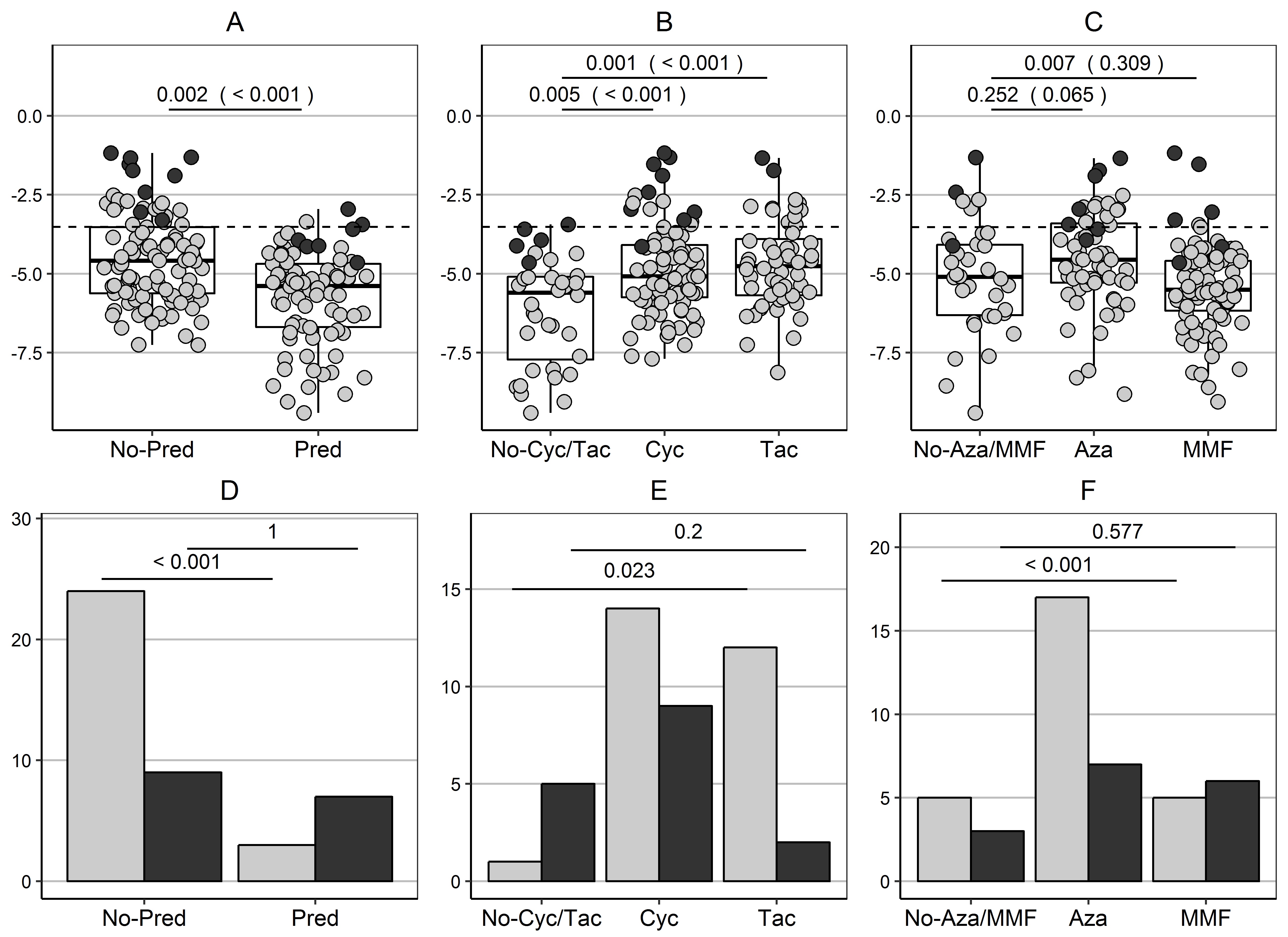
A multivariable linear regression model revealed that 27% of the variability of IGKV4.1 expression was explained by IS drug intake. We can confirm that prednisolone and CNIs intake have an effect on IGKV4.1 gene expression even after adjustment for intake of other IS drugs (Figure-1A,B). The tolerance selection was determined by drug intake, with considerably less patients on prednisolone and predominance of patients on CNI and azathioprine (grey bars in Figure-1 D,E,F).
We then performed drug-adjustment of gene expression. IGKV4.1 expression remained higher in tolerant patients (p=0.002) and the separation improved, with only 16 stable patients (9%) identified as potentially tolerant (black dots Figure-1 A,B,C), while the classification of stable KTRs as tolerant was no longer directed by immunosuppressants (black bars Figure-1D,E,F). The development of tolerance-associated signatures on the basis of unadjusted gene expression could have profound effect on the classification of stable patients as potentially tolerant.
With this data, we demonstrate how adjustment for IS-drug intake does not obliterate the contribution of genes to tolerance, when this exists; but it does indeed remove the effects ascribable to pharmacological immunosuppression and, thus, reveals underlying tolerance characteristics.
Consequently, we would argue that IS regimens do affect the expression of many genes (although not all) and require adequate investigation. When IS are, indeed, altering the expression of signature genes, investigators should adjust for IS-drug intake.
MRC (grants G0801537/ID: 88245 grants and Medical Research Council (MRC) Centre for Transplantation, – MRC grant no. MR/J006742/1) . Guy’s and St Thomas’ Charity (grants R080530 and R090782). . EU-FP7 Grant agreement no HEALTH-F5–2010–260687: The ONE Study. FP7-HEALTH-2012-INNOVATION-1 project number 305147: BIO-DrIM.. Funds/support by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. . All study centres in the consortium in UK received funding from Clinical Research Networks, study portfolio number 7521..
