C1q Assay to Determine Complement Fixing of Anti-Human Leukocyte Antigen Antibodies, but not Immunoglobulin Subclass Identification, Allows Better Prediction of Kidney Allograft Outcomes in an Asian Population
Rachel Teo1,2, Angeline Goh1, Robynne Koh2, Lai May Ling3, Yue Gu2, Paul MacAry2, Anantharaman Vathsala1,2.
1National University Centre for Organ Transplantation, National University Hospital, Singapore, Singapore; 2Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore; 3Health Sciences Authority, Singapore, Sinagpore, Singapore
Background. Anti-HLA antibodies play a key role in chronic antibody mediated rejection. Donor-specific anti-HLA antibodies (DSAs) in particular, are detrimental to allograft survival. The current Luminex platform using single antigen beads is highly sensitive and specific, but not entirely predictive. C1q binding and IgG subclass determination may be more useful for prediction of allograft outcomes. We aim to characterise prevalent DSAs and cross-reactive groups (CREGs) and hypothesise that this may improve immunological risk stratification for prediction of allograft outcomes in an Asian cohort of kidney transplant recipients.
Methods. We conducted a retrospective study on 58 patients who have prevalent DSAs/CREGs of mean fluorescent intensity (MFI) >3000. Sera were tested with Single Antigen Beads (SAB) in the Luminex® C1q and IgG subclass assays. IgG subclasses were identified using isotype specific secondary antibodies (Southern Biotech®). Analysis to examine primary outcomes of antibody-mediated rejection (ABMR), proteinuria (eTUP >0.3g/day) and graft dysfunction (eGFR<30ml/min) was based on immunodominant DSA/CREG (iDSA/CREG). We also performed verification of current available solid phase assays using engineered anti-A*11:01 antibody specific IgG 1 to 4 subclasses generated from a Humanyx® library. The anti-A*11:01 antibody was demonstrated to bind to SAB in the Luminex assay and was also validated in complement dependent cytotoxicity and flow cytometric cross matches against HLA A*11:01 donor cells.
Results and Discussion. In our cohort with DSA/CREG of MFI >3000, 18.6% had ABMR. In patients with C1q-binding iDSA/CREG, 33.3% had ABMR, compared to 13.6% with non-C1q binding iDSA/CREG, showing a trend towards but not reaching statistical significance (p=0.08). Combining C1q-binding and DSA/CREG MFI >3000 detected a higher rate of ABMR (33.3% vs 18.6). IgG1 was the predominant subclass (97%) and seemed to confer greater complement fixing ability when present in combination with other subclasses than alone. However, this did not improve prediction of allograft outcomes.
Verification of solid phase assays for identification of IgG isotypes showed inconsistent detection of IgG subclasses, in particular for IgG2 and IgG3. IgG1 was detected on the C1q assay only at higher concentration of 10ug/ml, while IgG3 was poorly detected even at increased concentration. This indicates that the current available assays for Ig isotype detection may not be entirely reliable or reproducible.
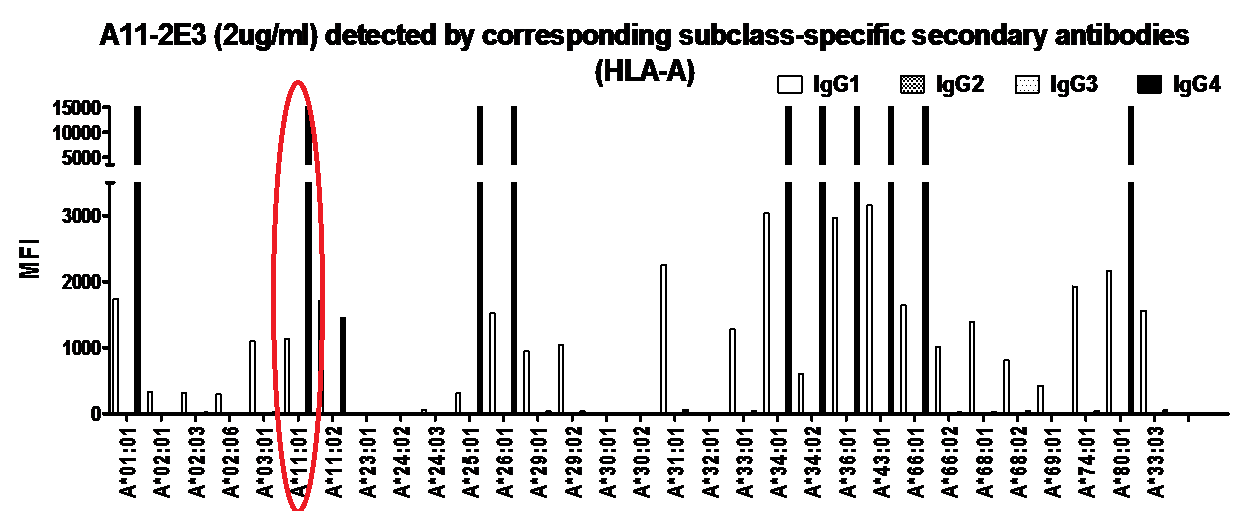
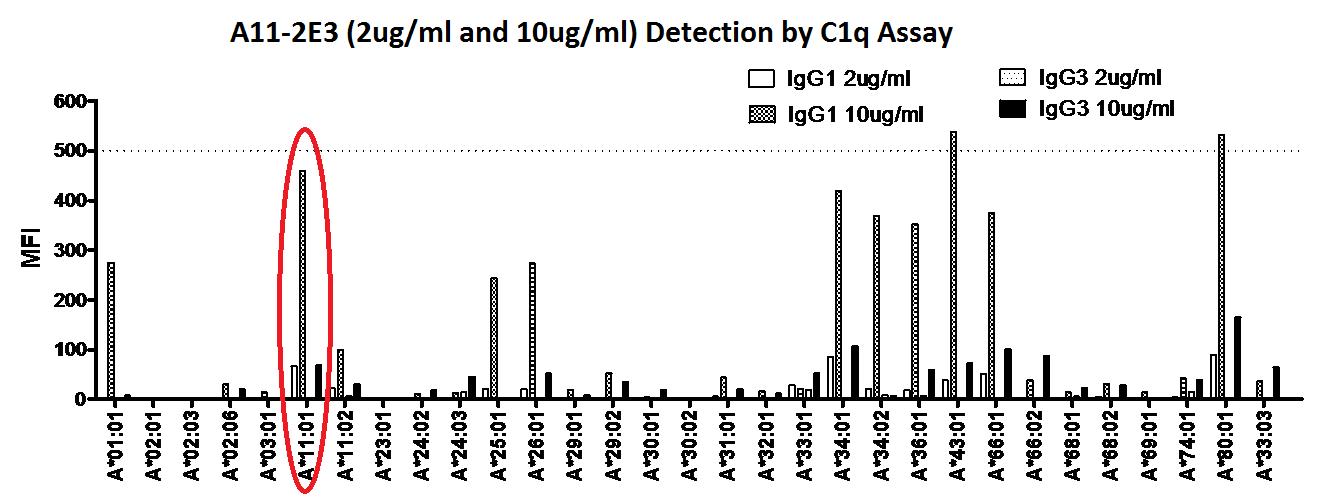
Conclusion. Based on current available assays, C1q binding ability in addition to MFI of anti-HLA antibodies improves prediction of ABMR, while IgG subclass does not. Further assay verifications are required to improve interpretation of current assays used for detailed characterisation of anti-HLA antibodies. Longitudinal studies are underway to determine their clinical utility.
Professor Kathryn Wood, University of Cambridge.
