
Prevascularization of the Subcutaneous Space Improves Survival of Transplanted Mouse Islets
Nicole Conkling1, Steven WIsel1, Gaetano Faleo1, Tejal Desai2, Peter Stock1, Qizhi Tang1.
1Surgery, University of California, San Francisco, San Francisco, CA, United States; 2Bioengineering and Therapeutic Sciences, University of California, San Francisco, San Francisco, CA, United States
Introduction: Beta cell replacement for the treatment of Type I diabetes has previously been limited by donor availability, invasive surgical techniques, and islet survival post-transplant. Transplantation into the subcutaneous space is ideal for graft accessibility and explant if necessary; however, prior investigations have shown that in comparison to other gold-standard sites (i.e., the kidney capsule), this location lacks the rich blood supply required to meet the high metabolic demands of islets. The aim of this study was to identify and demonstrate the efficacy of a novel prevascularization strategy to improve free islet graft survival in the subcutaneous space.
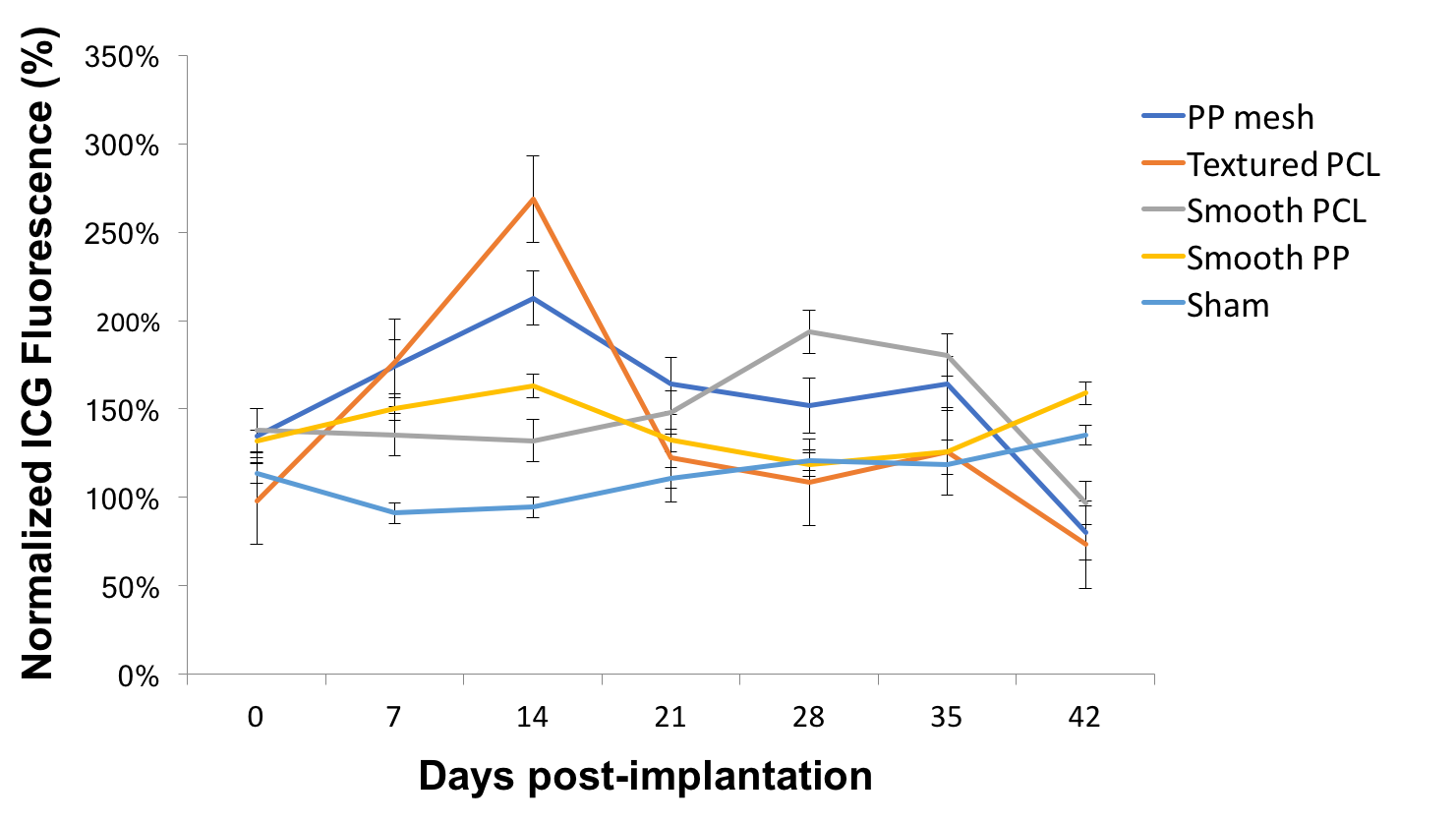
Methods: To investigate the ideal prevascularization device, four different materials were implanted into the subcutaneous space of B6 albino mice: polypropylene (PP) mesh, smooth PP, texturized polycaprolactone (PCL), and smooth PCL. Vascularity was assessed using indocyanine green fluorescence measurement at 1-week time points up to 6 weeks post implantation. At selected time points, mice were euthanized and specimens were examined using immunofluorescence histology.
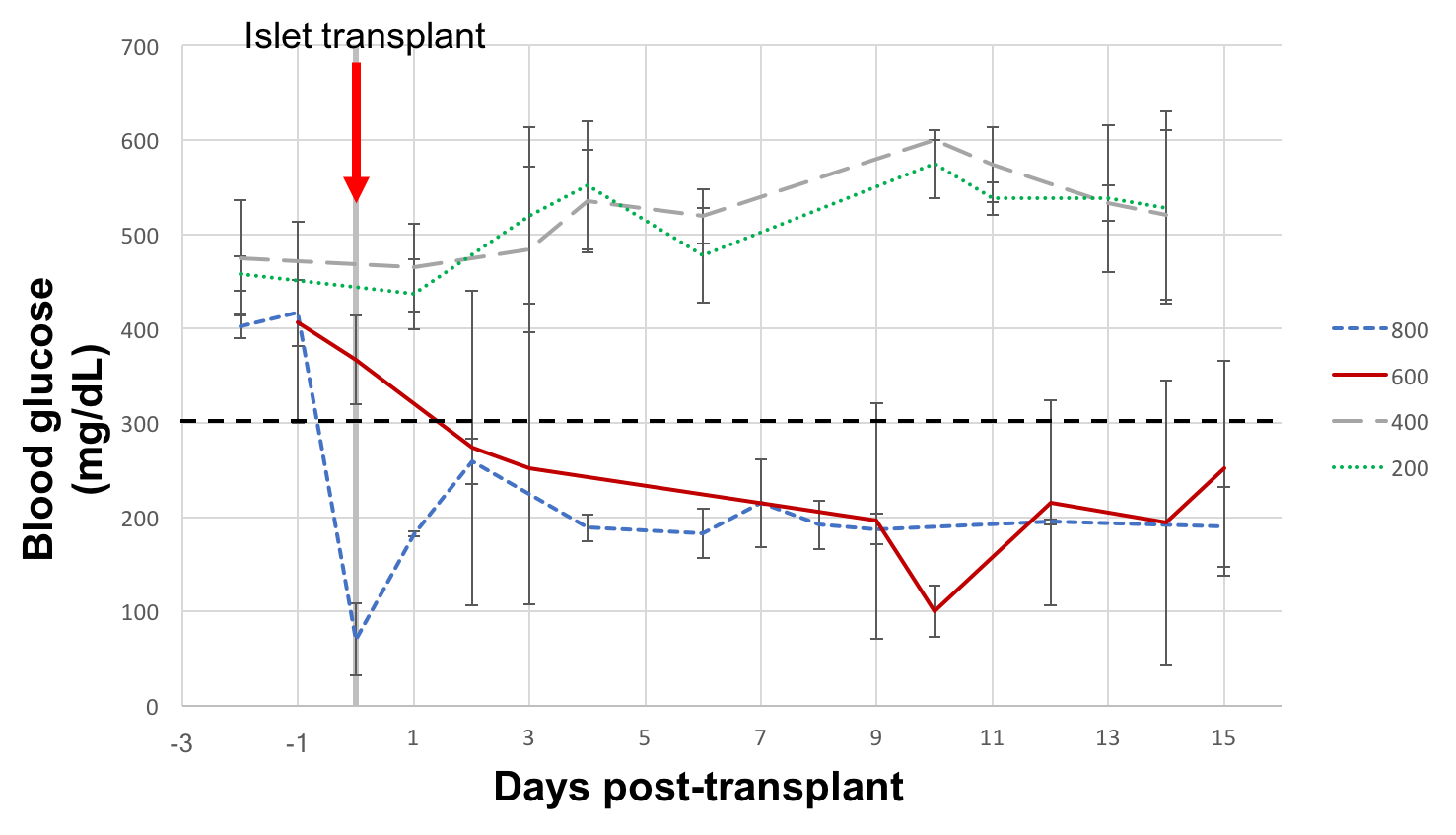
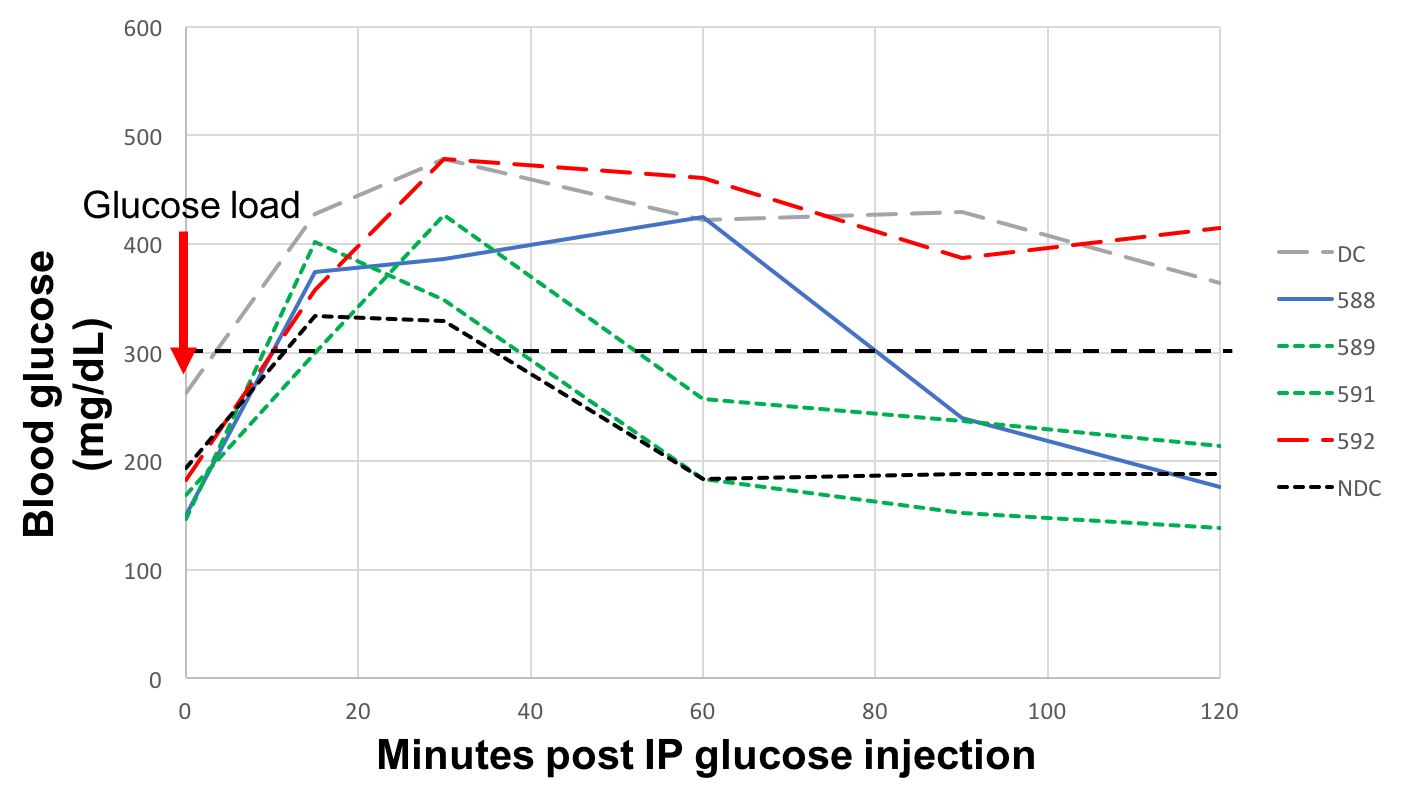
B6 mice that had been prevascularized for 2 weeks with textured PCL discs were made diabetic with intraperitoneal streptozosin injection (200 mg/kg). The prevascularization discs were removed, and the mice were then transplanted with 200, 400, 600, and 800 syngeneic mouse islets into the disc capsule. Blood glucose was measured for three weeks to monitor for diabetes cure. Intraperitoneal glucose tolerance test (IPGTT) was performed on transplant recipients to compare their function to diabetic and normal controls.
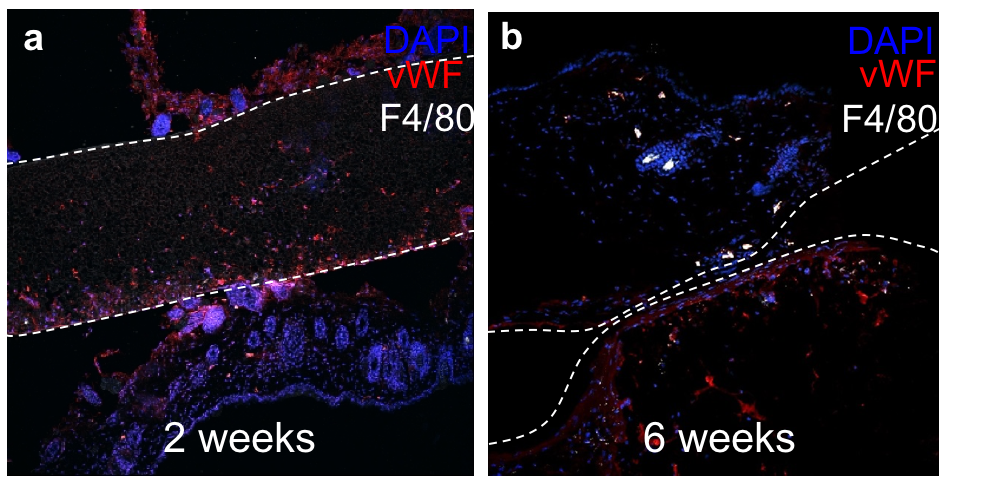
Results: When normalized to baseline fluorescence, the peak vascularity as measured by ICG fluorescence was determined for texturized PCL at the 2-week time point (Fig. 1). Histological analysis at 2 weeks (Fig. 2a) and 6 weeks (Fig. 2b) post-implantation demonstrated vascular ingrowth (vWF) and minimal foreign body reaction (macrophages, or F4/80).
Diabetes reversal was not observed for 200- or 400-islet transplant recipients. 100% diabetes cure was seen with 800-islet transplants, and partial cure (50%) was seen with 600-islet transplants (Fig. 3). When subjected to IPGTT at 2 weeks post transplant, the 600-islet group showed similar blood glucose kinetics to non-diabetic control in cured mice and impaired glucose tolerance in partially cured and failed mice (Fig. 4).
Conclusion: Texturized PCL induces improved vascularity in the subcutaneous space of mice while inciting minimal foreign body reaction. Free islets transplanted in the subcutaneous space of mice have improved survival in the prevascularized implant capsule, and allow for comparable function to non-diabetic controls. This strategy may represent a safe and effective translational pathway for the application of subcutaneous islet transplant in human subjects in the near future.
Research reported in this publication was supported in part by an NIAID T32 training grant from the National Institutes of Health under an award to the University of California, San Francisco (T32AI125222). .
