Master Regulator for Splenic Development, Tlx1 Contributes the Engraftment of the Transplanted Islets in the Spleen
Hiroyuki Takahashi1,2,3, Takeshi Itoh1,2, Hitomi Nishinakamura1, Naoaki Sakata1,2, Gumpei Yoshimatsu1,2, Taisuke Matsuoka1,2,3, Shohta Kodama1,2.
1Department of Regenerative Medicine & Transplantation, Faculty of Medicine, Fukuoka University, Fukuoka city, Japan; 2Center for Regenerative Medicine, Fukuoka University, Fukuoka city, Japan; 3Department of Gastroenterological Surgery, Faculty of Medicine, Fukuoka University, Fukuoka city, Japan
Introduction: Clinical islet transplantation (Tx) is a useful treatment for the severe diabetes mellitus. The liver has been recognized as an ideal transplant site for islet Tx due to the similarity of native insulin flow, however it has a disadvantage in poor transplant efficacy due to the active innate immune system. To establish an alternative transplant site, we have focused on the spleen, a component of portal circulation as same as pancreas and liver, and splenocytes promote immune tolerance which might prevent graft rejection. And we previously reported that Tlx1+ stem cells in the spleen regenerated into insulin-producing cells in the pancreas. This study aimed to show the effectiveness of intrasplenic islet Tx.
Materials and Methods: We tried to clarify the marginal numbers of islets for achieving normoglycemia using diabetic C57BL/6 mice, which underwent the transplantation of 25, 50, 100 or 200 islets equivalents (IEQs) into the liver (L), beneath the left kidney capsule (KC), or into the spleen (SP). Intraperitoneal glucose tolerance tests (IGTT) were performed at 50 days after Tx, and a splenectomy was done at 60 or 160 days. Plasma MCP-1, G-CSF and HMGB-1 levels at 6 hours after Tx was also measured using ELISA or MAGPIX systems. Next, we transplanted 25 IEQs into the spleen to figure out the minimum dose of islets under controlling insulin with transplantation of 100 IEQs beneath KC (SP25+KC100 or SP25 alone group). To assess the graft function in the spleen, the nephrectomy was performed at 240 days after Tx and the splenectomy was done at 290 days. Additionally, microarray was performed to detect the gene regulation associated with improving islet engraftment.
Results and Discussions: The marginal islets Tx number in L, KC, or SP or 200, 100, or 50 IEQs, respectively. 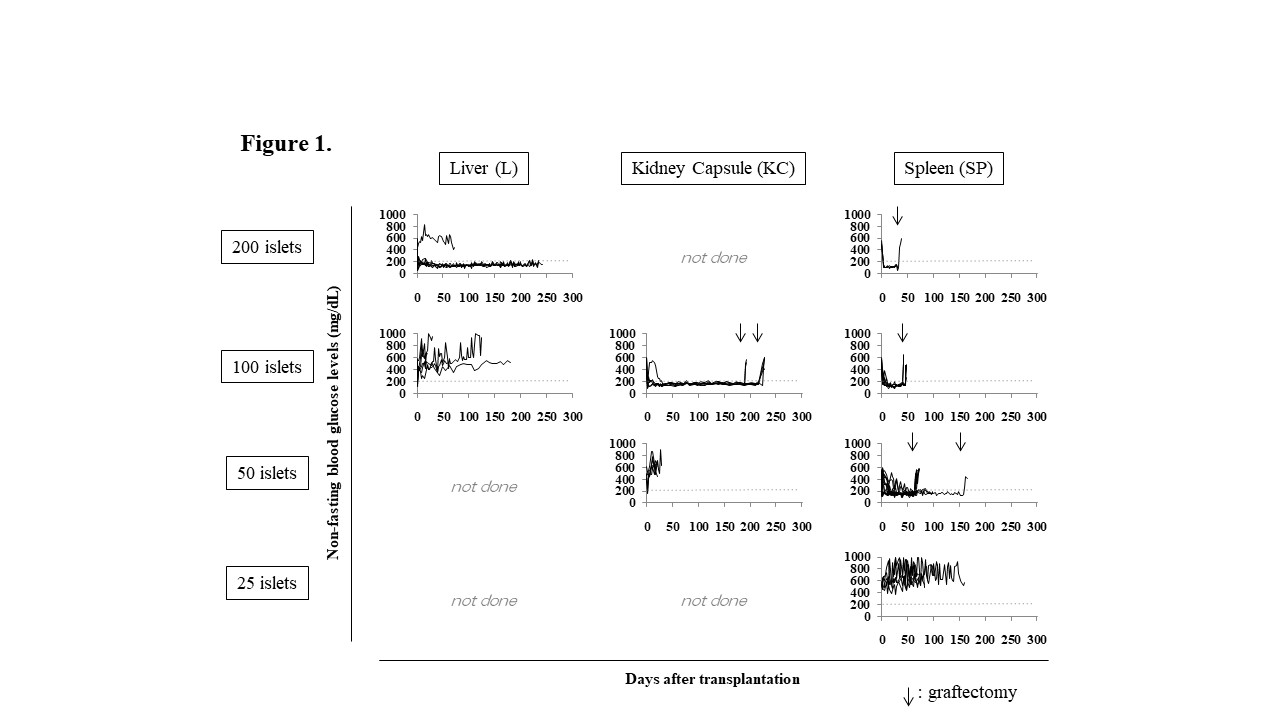 The pattern of glucose change in IGTT of the mice transplanted 50 IEQs into the SP (SP50) was comparable to that of naïve control mice. All the SP50 mice became hyperglycemia after splenectomy. All the plasma MCP-1, G-CSF and HMGB-1 levels in the SP50 mice were significantly lower (p < 0.05) than those of the mice received 200 IEQs into the L (43.4±11.1 pg/mL, vs. 77.0±40.2 pg/mL, 4,970.7±1,176.1 pg/mL vs. 7,862.6±1,023.4 pg/mL, 12.4±1.2 ng/mL vs. 27.7±6.6 ng/mL, respectively) indicating that reduction of inflammation was seen in the SP group. All SP25+KC100 mice (n = 11) became normoglycemia, and the eight of them remained normoglycemia even after nephrectomy.
The pattern of glucose change in IGTT of the mice transplanted 50 IEQs into the SP (SP50) was comparable to that of naïve control mice. All the SP50 mice became hyperglycemia after splenectomy. All the plasma MCP-1, G-CSF and HMGB-1 levels in the SP50 mice were significantly lower (p < 0.05) than those of the mice received 200 IEQs into the L (43.4±11.1 pg/mL, vs. 77.0±40.2 pg/mL, 4,970.7±1,176.1 pg/mL vs. 7,862.6±1,023.4 pg/mL, 12.4±1.2 ng/mL vs. 27.7±6.6 ng/mL, respectively) indicating that reduction of inflammation was seen in the SP group. All SP25+KC100 mice (n = 11) became normoglycemia, and the eight of them remained normoglycemia even after nephrectomy. 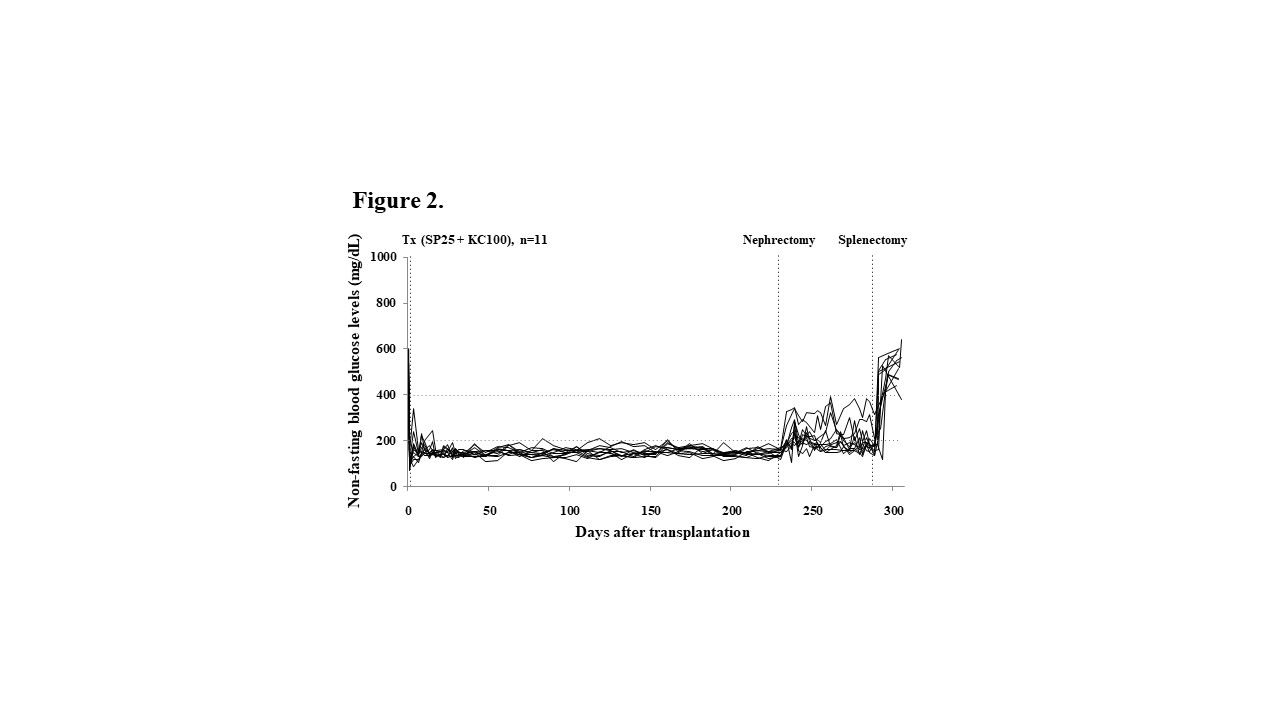 All of them became hyperglycemia after splenectomy that meant the islet graft in the spleen kept normoglycemia. Additionally, Tlx1-related genes including Rrm2b and Pla2g2d, which encode a transcription factor essential for the spleen development, were up-regulated following islet engraftment in the SP.
All of them became hyperglycemia after splenectomy that meant the islet graft in the spleen kept normoglycemia. Additionally, Tlx1-related genes including Rrm2b and Pla2g2d, which encode a transcription factor essential for the spleen development, were up-regulated following islet engraftment in the SP.
Conclusion: The spleen is an ideal transplant site for the islet to reduce inflammation and support the engraftment of islets.
