Renal Function Outcomes in De Novo Kidney Transplant Recipients Receiving Everolimus with Reduced-Exposure Calcineurin Inhibitor versus Mycophenolate with Standard-Exposure Calcineurin Inhibitor: Results from the TRANSFORM Study
Federico Oppenheimer1, Graeme Russ2, Ondrej Viklicky3, Rainer Oberbauer4, Valter Garcia5, Oliver Witzke6, Dirk Kuypers7, Romina Danguilan8, Ayan Das Gupta9, Peter Bernhardt10, Claudia Sommerer11.
1Hospital Clinic de Barcelona, Barcelona, Spain; 2Royal Adelaide Hospital, Adelaide, Australia; 3IKEM, Prague, Czech Republic; 4Medical University of Vienna, Vienna, Austria; 5Santa Casa de Misericordia do Porto Alegre, Porto Alegre, Brazil; 6Universitätsklinikum Essen, Essen, Germany; 7Gasthuisberg University Hospital, Leuven, Belgium; 8National Kidney and Transplant Institute, Quezon City, Philippines; 9Novartis Healthcare Pvt. Ltd, Hyderabad, India; 10Novartis Pharma AG, Basel, Switzerland; 11Universitätsklinikum Heidelberg, Heidelberg, Germany
Introduction: Existing evidence suggests that de novo initiation of everolimus (EVR) with reduced-exposure calcineurin inhibitor (rCNI) preserves renal function while maintaining efficacy and safety in kidney transplant recipients (KTxRs). TRANSFORM (NCT01950819) is the largest study conducted in de novo KTxRs to evaluate the benefit of EVR+rCNI compared to mycophenolate (MPA) with sCNI. Here, we present renal function outcomes.
Method: This is a 24-month (M), phase IV, multicentre, open-label study in de novo adult KTxRs randomised within 24 h of Tx to either EVR+rCNI (N=1022; EVR trough level [C0]: 3-8 ng/mL; tacrolimus [TAC] C0: 4-7, 2-5, and 2-4 ng/mL or cyclosporine [CsA] C0: 100-150, 50-100, and 25-50 ng/mL from Day 1(D1)-M2, M3-M6, and M7-M24, respectively) or MPA+sCNI (N=1015; MPA [1.44 g/day of EC-MPS or 2.0 g/day of MMF]; TAC C0:8-12, 6-10, and 5-8 ng/mL or CsA C0: 200-300, 150-200, and 100-200 ng/mL from D1-M2, M3-M6, and M7-M24, respectively). All patients received basiliximab or rabbit antithymocyte globulin induction and steroids. Renal function assessments included evaluation of eGFR in overall population, Asian sub-population and by CNI type at M12.
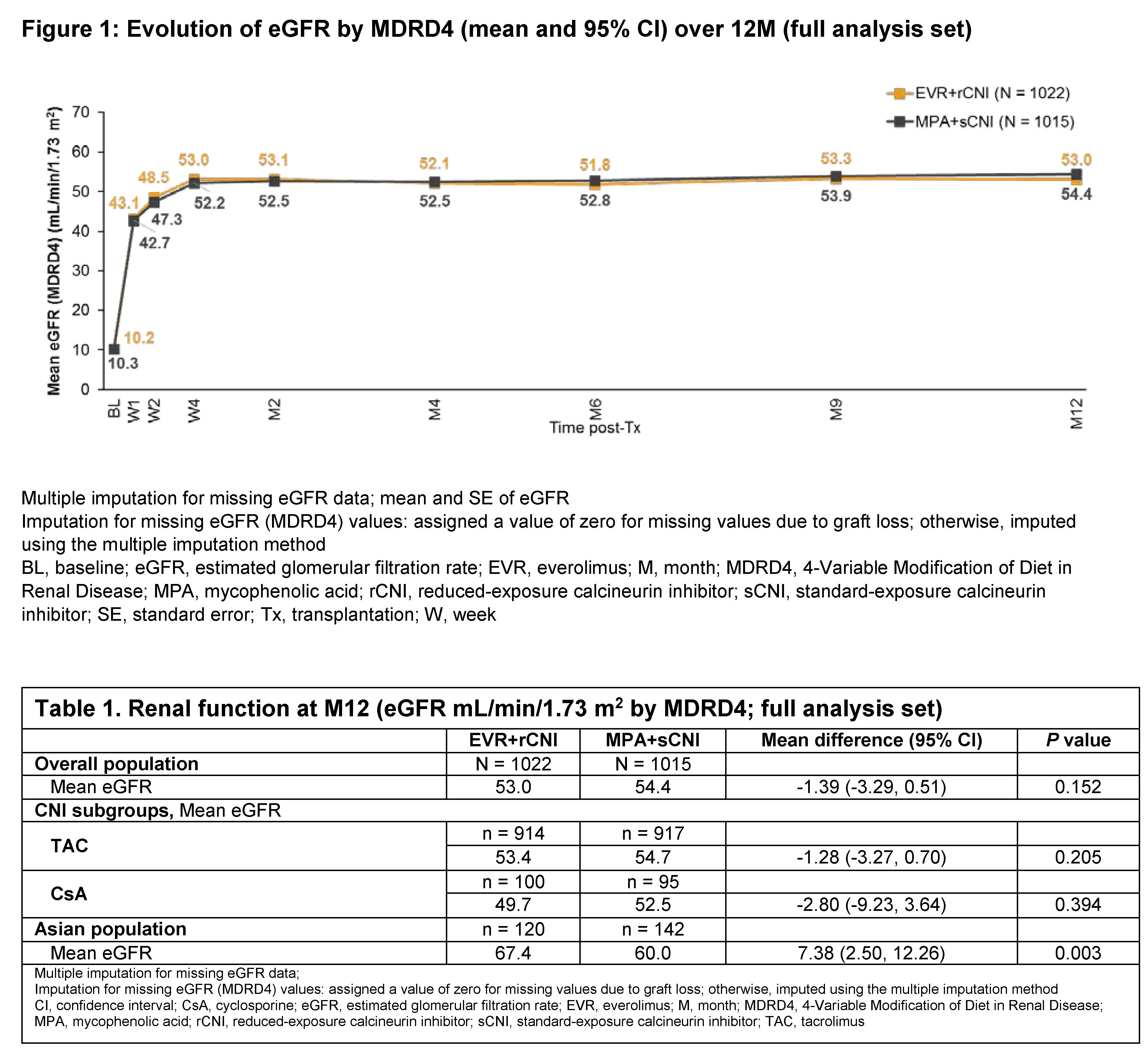
Results: A total of 925 (90.5%) patients in EVR+rCNI arm and 922 (90.8%) in MPA+sCNI arm completed 12M study. Demographics and baseline characteristics were balanced between arms. At M12, mean EVR C0 in EVR+rCNI arm was 5.5 ng/mL with 86.3% of patients within target C0 range. At M12, 40.0% and 47.6% of patients were above the target C0 of TAC and CsA in EVR+rCNI arm, respectively, whereas 62.1% of patients had TAC C0 and 71.6% had CsA C0 within target range in MPA+sCNI arm. At M12, mean eGFR was similar between the EVR+rCNI (53.0 mL/min/1.73 m2) and MPA+sCNI (54.4 mL/min/1.73 m2) arms (Figure 1). Notably, in Asian sub-population, M12 mean eGFR was significantly higher in EVR+rCNI (n = 120) vs MPA+sCNI (n = 142) arm (67.4 vs 60.0 mL/min/1.73 m2; difference: 7.4 mL/min/1.73 m2; P=0.003; Table 1). Frequency of patients with eGFR <50 mL/min/1.73 m2 was similar in EVR+rCNI (45.4%) and MPA+sCNI arms (42.2%; difference: 3.2% [−1.3-7.6]). At M12, mean urinary protein/creatinine ratio was comparable between EVR+rCNI (298.56 mg/g) and MPA+sCNI (233.80 mg/g) arms. Most patients in EVR+rCNI (85.9%) and MPA+sCNI arms (92.0%) had mild proteinuria (30-<500 mg/g).
Conclusion: Despite CNI C0 being above the target range in most patients in EVR+rCNI arm, renal function was comparable in both treatment arms in the overall population at M12. Long-term effects of the EVR+rCNI regimen on renal function in this population will be further confirmed from the 24M results.