
Co-Transplant of Parathyroid Gland and Stem Cell-Derived Insulin-Producing Cells Enhances Graft Survival through Release of Pro-Angiogenic and Pro-Survival Factors
Gaetano Faleo1, Casey Ward1, Audrey Parent3, Gopika Nair3, Wenhan Chang2, Peter Stock1, Matthias Hebrok3, Qizhi Tang1,3.
1Transplantation Research Lab, UCSF, San Francisco, CA, United States; 2San Francisco Veteran Affairs Medical Center, VA, San Francisco, CA, United States; 3Diabetes Research Center, UCSF , San Francisco, CA, United States
Introduction: Ischemia dramatically impairs survival and function of islets after transplantation. Human Stem Cell-derived Beta Cells are currently being tested in clinical trials for replacement therapy. Although stem cell technology represents an unlimited source of beta cells, it is paramount to reduce the effects of ischemia to optimize outcome. Parathyroid glands (PTG) are known to engraft in the subcutaneous and intra-muscular sites with high success rate. The objective of this study is to investigate if PTG can protect beta-cell grafts. We hypothesize that PTG protect beta cells by both accelerating neo-vasculogenesis and by producing tropic factors that sustain beta cell viability before revascularization.
Methods: Stem Cell-derived Beta Cells were produced from a human embryonic stem cell line with a GFP transgene knocked into the insulin locus and a constitutively expressed luciferase transgene. Insulin-producing GFP+ cells were enriched by FACS and reaggregated to form enhanced beta clusters (eBCs). eBC were transplanted under the subcutaneous space (SQ) of NSG mice and graft mass was monitored using bioluminescence imaging. Human PTG were finely cut and co-transplanted with eBC. PTG-derived CD34+ and CD34- cells were FACS purified, cultured for 24 hours and supernatants were assessed for cytokines using Luminex. Effects of PTG-derived factors on eBCs viability after challenges of hypoxia and nutrient deprivation were assessed in vitro.
Results and Discussion: eBC graft mass decreased by 60% in two weeks after transplant under the SQ. However, when eBC grafts were co-transplanted with PTG, bioluminescent signal increased up to 150% (Figure 1 A and B). 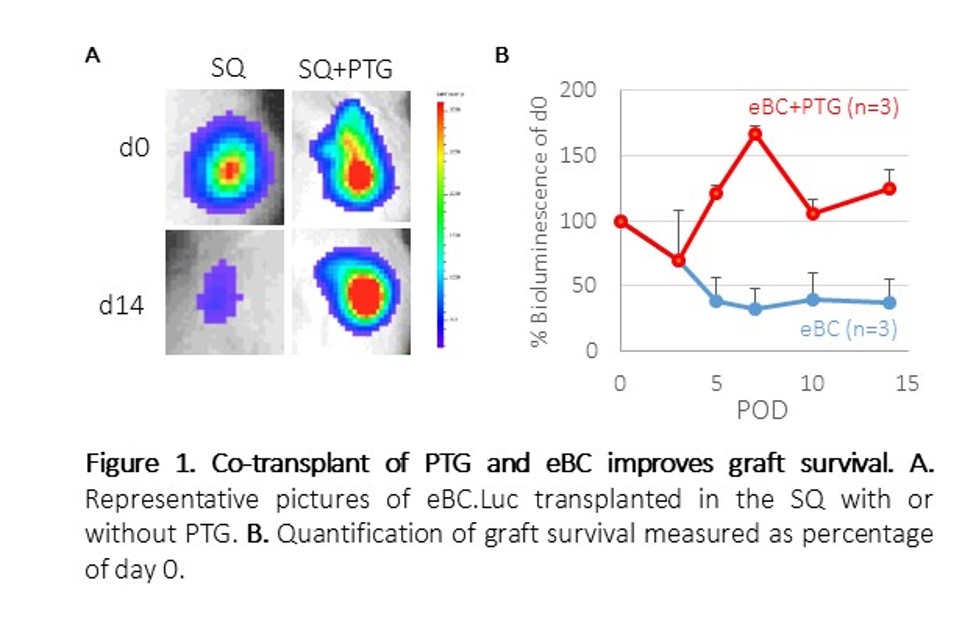
We further investigated the mechanisms of PTG protection. We found PTG-derived CD34+ cells produced CCL2, CXCL12, VEGF-A, and PDGF-AA (Figure 2), which have all been previously implicated in neo-vasculogenesis. 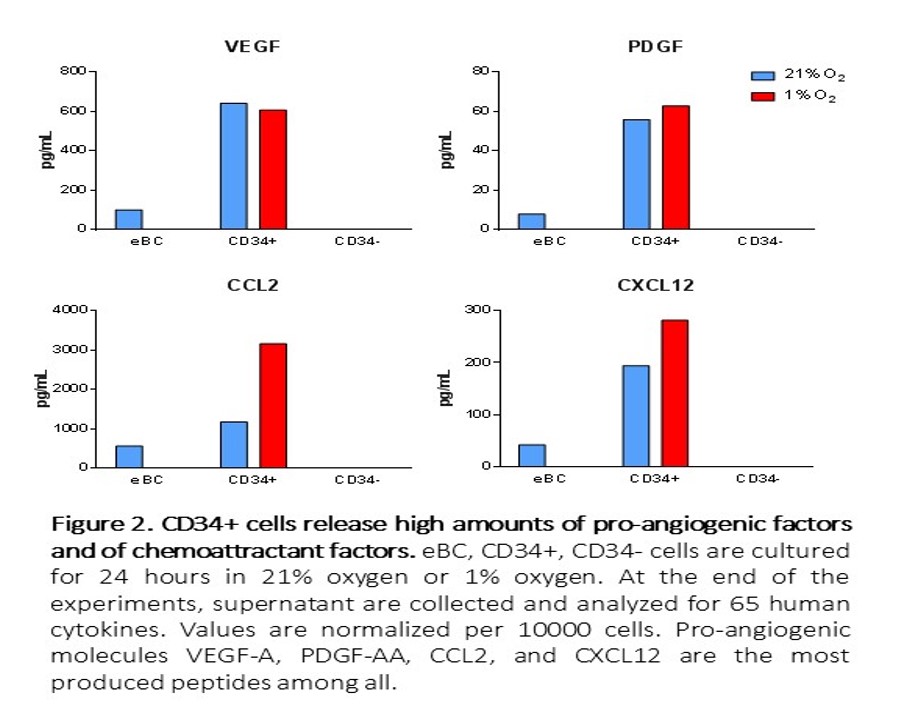
Consistently, PTG co-transplant led to a dramatic increase in the ingrowth of CD31+ cells. Additionally, PTG secreted parathyroid hormone (PTH), parathyroid hormone-related protein (PTHrP), and γ-aminobutyric acid (GABA). These factors improved eBC survival during in vitro-simulated ischemia challenge using nutrient-deprivation and/or hypoxia (Figure 3). 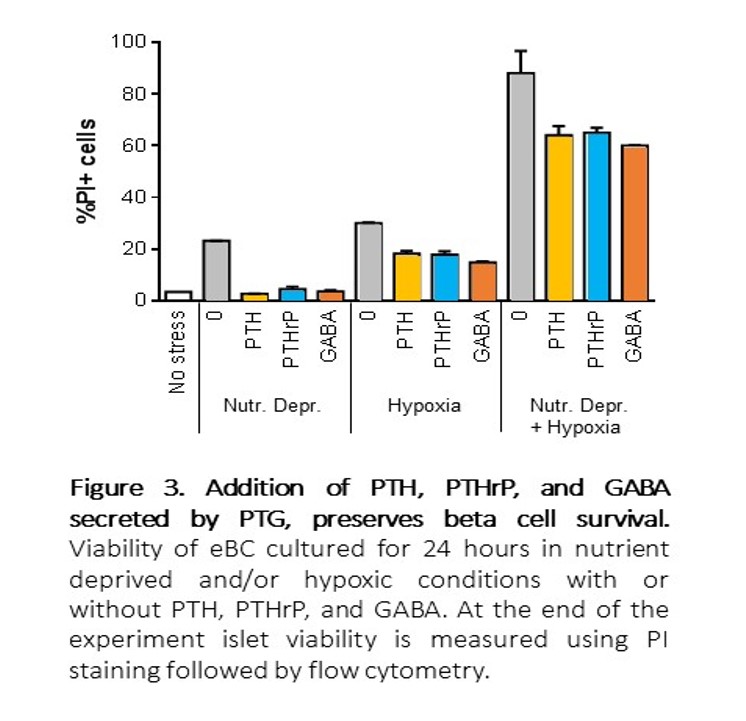
Conclusions: Our results show that PTG co-transplantation preserves beta cell graft survival. PTG-derived CD34+ cells promote neovascularization of eBC grafts by producing angiogenic factors. PTG may also have direct cytoprotective effects on eBCs by producing PTH, PTHrP, and GABA. These properties of PTG may be harnessed to minimize ischemic injury to eBC after transplant to realize the therapeutic potential of stem cell-based therapy for diabetes.
