Rebeca C. Arroyo Hornero, United Kingdom has been granted the TTS Young Investigator Scientific Award
Co-Stimulatory Modulation of Human Regulatory T cells for Enhanced Immunotherapy
Rebeca Arroyo Hornero1, Kathryn Wood1, Joanna Hester1, Fadi Issa1.
1Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom
Introduction: Regulatory T cells (Treg) are a promising therapy in transplantation. However, current Treg expansion protocols lead to a heterogeneous cell product that contains cells with potentially detrimental pro-inflammatory properties. Ligation of CD27 on T cells with CD70 on APCs promotes T cell function and survival, but impairs Th17 activity. We have previously shown that CD27 expression on human Tregs correlates closely with suppressive potency. In the current study, we hypothesised that modulation of the CD27/CD70 pathway may allow for the generation of a purer population of Tregs with enhanced suppressive properties.
Materials and Methods: CD4+CD127low/-CD25+ Tregs were flow sorted from PBMCs and expanded in vitro with αCD3/αCD28 stimulation and IL-2. Expanded cells were sorted according to their expression of CD27 and CD70 and further expanded for 14 days. Expanded Treg subsets were examined in the presence or absence of αCD27/αCD70 neutralising antibodies. Phenotype was analysed by flow cytometry. Cytokine production was measured intracellularly and extracellularly by flow cytometry and ELISA. Suppressive function was assessed both in vitro and in vivo in immunodeficient mice.
Results and Discussion: Human Tregs differentially altered their expression of surface CD27 and CD70 after in vitro expansion, resulting in two distinct subpopulations. Functional in vivo and in vitro assays revealed that suppressive capacity was confined to the CD27+CD70- population. Conversely, CD27-CD70+ Tregs lost their ability to inhibit T cell proliferation. The pre-incubation of total Tregs or CD27-CD70+ Tregs with an αCD70 blocking antibody significantly increased their suppressive potency, suggesting that the CD27/CD70 pathway plays a direct role in Treg activity. Moreover, CD27-CD70+ Tregs, but not CD27+CD70- Tregs, produced high levels of pro-inflammatory cytokines and promoted T cell proliferation in the absence of external co-stimulation.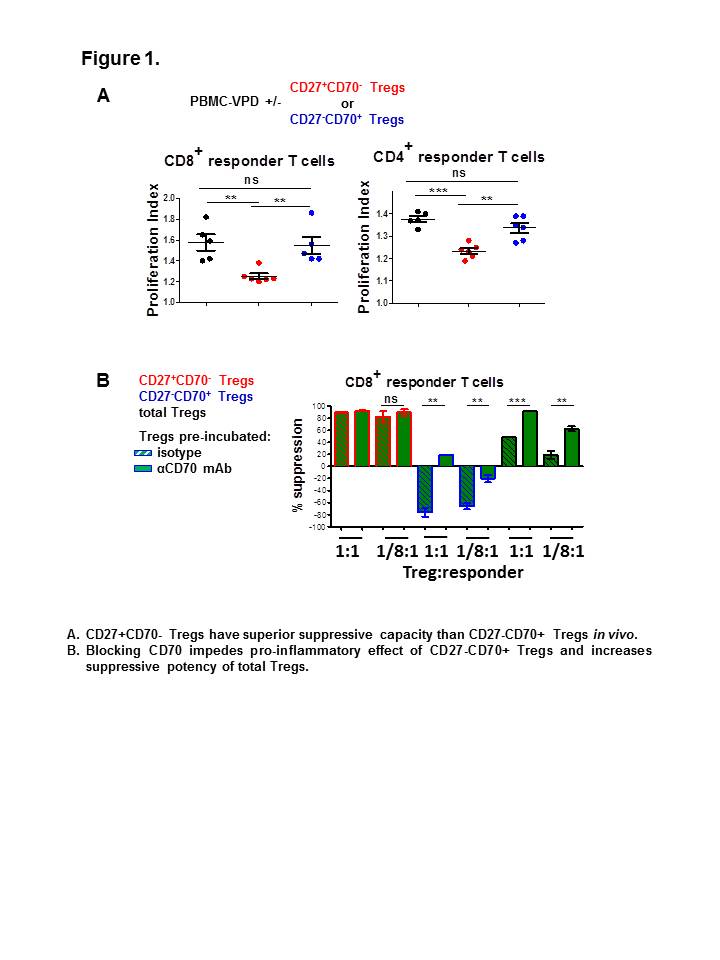 Conclusion: Selection on CD27+CD70- after expansion allows for the identification of functionally potent human Tregs. Using these markers eliminates a major component of unstable, proinflammatory cells in the expanded Treg product. This additional selection step may easily be incorporated into current GMP production methods to enhance the efficacy and safety of Treg therapy. In addition, there is an opportunity for the direct modulation of the CD27/CD70 pathway to enhance Treg function.
Conclusion: Selection on CD27+CD70- after expansion allows for the identification of functionally potent human Tregs. Using these markers eliminates a major component of unstable, proinflammatory cells in the expanded Treg product. This additional selection step may easily be incorporated into current GMP production methods to enhance the efficacy and safety of Treg therapy. In addition, there is an opportunity for the direct modulation of the CD27/CD70 pathway to enhance Treg function.
