Adherence to Immunosuppressive Medications in Renal Transplant Recipients – Different Tools Capture different Patients
Marte Gustavsen1,2, Kjersti Lønning1, Karsten Midtvedt PhD1, Anna V Reisæter PhD1, Anders Åsberg PhD1,2.
1Department of Transplantation Medicine, Oslo University Hospital, Rikshospitalet, Oslo, Norway; 2Department of Pharmaceutical Bioscience, University of Oslo, School of Pharmacy, Oslo, Norway
Background: Adherence to immunosuppressive therapy is important for patient and graft survival. An optimal adherence tool should be easily applicable in routine clinical setting. The primary objective of this study was to validate tools and tool combinations, suitable for annual routine capture of adherence data in renal transplant recipients (RTxR).
Method: A total of 300 RTxR using tacrolimus (Tac) as part of their immunosuppressive therapy were included in a single center open randomized prospective trial. Two thirds of the recipients were included within 4 weeks after transplantation and followed for 1 year (Group A). The other third was investigated cross-sectional at a 1-year post RTx control (Group B). The “Basel Assessment of Adherence to Immunosuppressive Medication Scale” (BAASIS) questionnaire capture taking and timing adherence, and was used to assess self-report adherence at 8 weeks and 1 year. In conjunction to the 8 weeks and 1-year investigation the treating health care provider (i.e. physician or nurse) scored patients adherence. Tac trough concentrations with corresponding doses were collected during the whole study period. The recipients were grouped as adherent (Ad)/non-adherent (NoAd) according to the different tools (Table 1).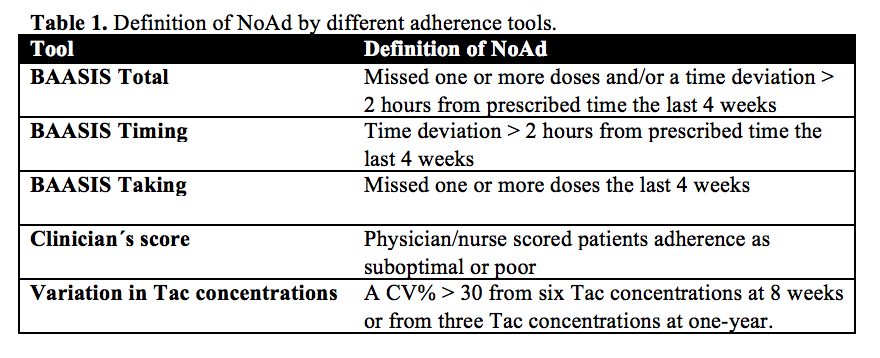
Results: Mean age at inclusion was 55 (22-80) years, 73% males and 31% received a kidney from a living donor. The BAASIS questionnaire showed an increasing number of NoAd recipients with increasing time after transplantation: 9% at 8 weeks and 28% at 1 year. The majority of NoAd had a time-deviation over 2 hours from prescribed time (7% 8 weeks, 25% 1 year). NoAd clinician´s score was 3% at 8 weeks and 9% at 1 year, while variation in Tac concentrations captured 3% and 12% as NoAd respectively. The tools showed some degree of overlap, but captured generally different NoAd populations. A combination of BAASIS, clinician´s score and Tac variation defined 38% of the recipients as NoAd at 1 year (Table 2). But when not considering recipients that only had a time deviation > 2 hours as NoAd, the combination defined 22%.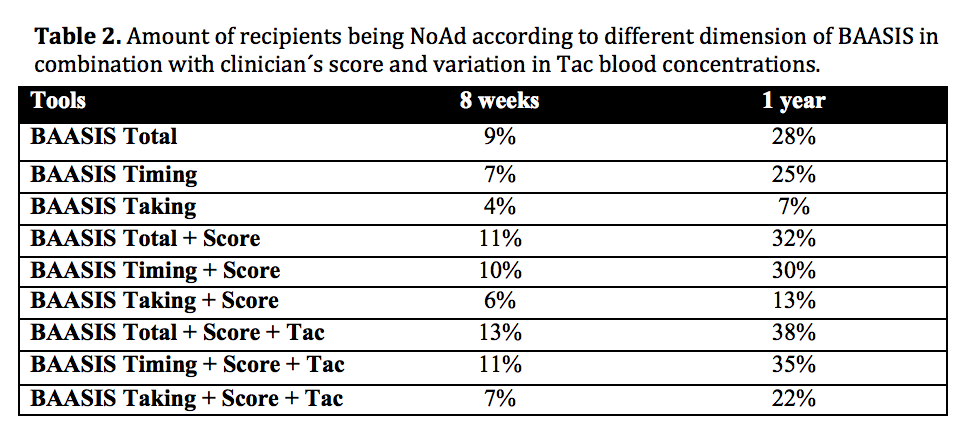
Conclusion: NoAd increased with time after transplantation, mainly due to intake of medications > 2 hours from prescribed time. The combination of BAASIS, clinician´s score and Tac variation identifies to a large degree different proportion of risk patients. The combination of these tools is possible to use on an annual basis. The 2 hours time deviation in BAASIS as a single marker for NoAd seems to be too rigid, and the focus should mainly be on missed doses. In the investigated population about 1 out of 5 patients showed true NoAd at 1-year post-engraftment.
