
In Kidney Allografts Inflammation in Scarred Areas is not a Reflection of Chronic Active T Cell-Mediated Rejection
Philip Halloran1,2, Jessica Chang1, Konrad S Famulski1,3.
1Alberta Transplant Applied Genomics Centre, Edmonton, AB, Canada; 2Medicine, University of Alberta, Edmonton, AB, Canada; 3Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada
Introduction: Inflammation in fibrotic renal parenchyma (i-IFTA) is associated with decreased graft survival. It has been postulated recently that i-IFTA represents chronic active T cell-mediated rejection (TCMR). The alternative view is that i-IFTA represents an active response to wounding caused by many forms of nephron injury, and was strongly associated with acute kidney injury transcripts (1). We analyzed our set of indication biopsies that had been scored for i-IFTA to determine the frequency of active histologic or molecular TCMR to determine the role of TCMR in i-IFTA.
Materials and Methods: In INTERCOMEX (Clinicaltrials.gov NCT01299168), 234 indication biopsies were assessed by a pathologist for i-IFTA, blinded to molecular results. Gene expression was measured by Affymetrix microarrays. Using our Molecular Microscope® diagnostic system (MMDx), based on the gene expression data, we conducted the archetypal analysis (a meta-classifier based on seven molecular classifiers) and assigned biopsies to three molecular diagnoses: Antibody-mediated rejection (ABMR), TCMR and no rejection. We also assigned molecular scores of acute kidney injury (AKI) and T cell burden (QCAT).
Results: We assigned 234 biopsies to three groups: group 1, without scaring and inflammation (ci=0, i-IFTA=0); group 2, with scaring and no inflammation (ci>0, i-IFTA=0); and group 3, with scarring and i-IFTA (ci>0, i-IFTA>0). The third group had the highest frequency of either histologic or MMDx diagnosis of ABMR (31% and 37%, respectively), whereas TCMR (histologic or molecular) was uncommon (8% and 7%, respectively, Table 1). 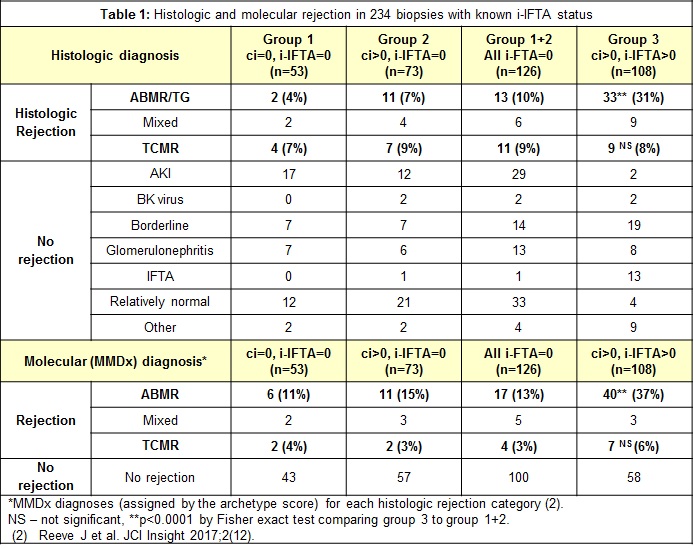 Histologic and molecular ABMR are both increased in group 3/i-IFTA compared to group 1+2, but neither histologic nor molecular TCMR is increased. Moreover, most biopsies with i-IFTA had neither histologic TCMR/mixed (90/108=83%) nor molecular TCMR/mixed (98/108=91%), but some did have a disease process diagnosed e.g. glomerulonephritis.
Histologic and molecular ABMR are both increased in group 3/i-IFTA compared to group 1+2, but neither histologic nor molecular TCMR is increased. Moreover, most biopsies with i-IFTA had neither histologic TCMR/mixed (90/108=83%) nor molecular TCMR/mixed (98/108=91%), but some did have a disease process diagnosed e.g. glomerulonephritis.
Frequency of graft failures was also highest in the third group (Table 2) and the graft loss was 24% in ABMR, 8% in mixed rejection and 4% in TCMR by histology, and 48%, 2% and 8% by the MMDx diagnosis, respectively. While group 3/i-IFTA had significantly more graft loss, AKI, T cell burden, and molecular ABMR, it did not have higher TCMR scores than group 1+2, and the mean TCMR classifier scores were below the 0.10 classifier cut off for positivity (Table 2). 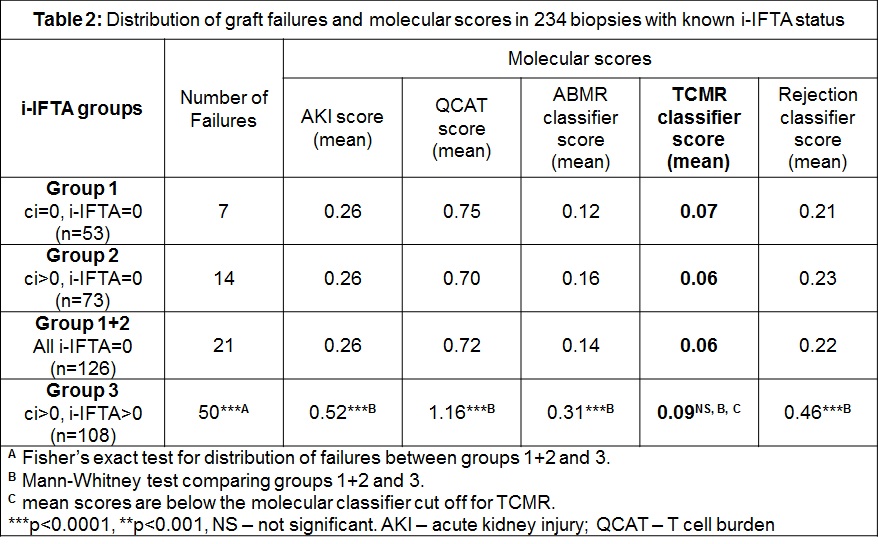
Conclusion. We conclude that most biopsies with i-IFTA have no molecular rejection, and in particular, seldom have molecular TCMR. i-IFTA is associated with ABMR, but not TCMR when both are diagnosed by histology and MMDx, and ABMR also drives graft failures in rejecting kidneys. Inflammation in scared areas reflects the tissue response to ongoing injury, but it is not related to chronic active TCMR.
(1) Famulski KS et al. Am J Transplant 2013;13(3):634-44.
