Enhancement of Intrinsic Apoptosis via Bcl-2 Inhibition Promotes Induction of Robust Mixed Chimerism and Renal Allograft Tolerance without Myelosuppression in Nonhuman Primates
Tetsu Oura1, Abbas Dehnadi1, Hajime Sasaki1, Ivy Rosales1, A. Bendict Cosimi1, Pietro Cippa2, Thomas Fehr2, Tatsuo Kawai1.
1Transplant Center, Massachusetts General Hospital, Boston, MA, United States; 2Medicine, University of Zurich, Zurich, Switzerland
Background: Successful induction of mixed chimerism (MC) and renal allograft tolerance has been achieved in both nonhuman primate (NHP) and man following conditioning that included 3.0 Gy total body irradiation (TBI). The significant myelosuppression resulting from this treatment has led to the search for alternative strategies for wide clinical application. We have, therefore evaluated the novel approach of B cell lymphoma-2 (Bcl-2) inhibition for specific enhancement of lymphocyte apoptosis.
Methods: In Group 1 (G1), four NHP recipients received combined kidney and bone marrow transplantation (CKBMT) following reduced dose (1.5 Gy) TBI, thymic irradiation (TI, 7.0 Gy), ATG and the Bcl-2 inhibitor (ABT-199: 10 mg/kg x 11 on days -4 to 6). After CKBMT, anti-CD154 mAb (20 mg/kg x 4) and cyclosporine for 28 days were administered. Two recipients received the same regimen without TBI (G2) and three without TI (G3). The results were compared with recipients treated with the standard 3.0 Gy TBI (G4: n=8) or with 1.5 Gy TBI (G5: n=2) but without ABT-199.
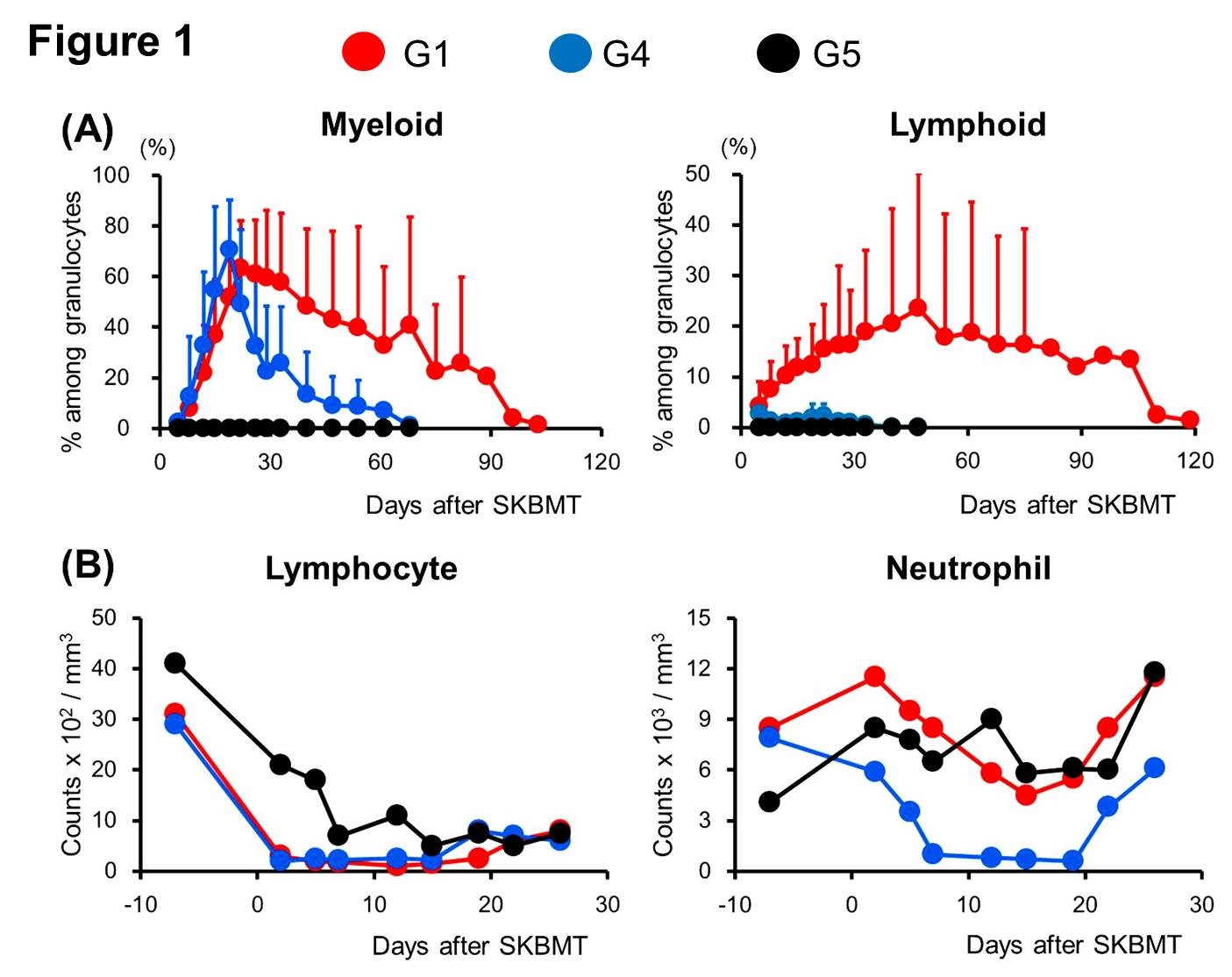
Results: 4/4 G1 recipients developed markedly higher and more prolonged MC (Fig. 1A) with excellent lymphocyte depletion but without neutropenia (Fig. 1B) in contrast to G4 recipients (3.0 Gy TBI without ABT-199). Recipients in G2 (no TBI) failed to develop MC, indicating that minimal TBI is necessary even with ABT-199 for induction of MC. G1 recipients achieved long-term allograft survival (313, >177, >100, and >65 days) without rejection, while all recipients in G2 (no TBI), G3 (no TI) or G5 (1.5 Gy without ABT-199) developed rejection.
Conclusion: An FDA approved Bcl-2 inhibitor, ABT-199 with low-dose TBI and essential TI induced robust MC and long-term renal allograft acceptance without myelosuppression in NHP CKBMT. Enhancement of intrinsic apoptosis with Bcl-2 inhibition is a promising strategy to achieve robust MC and renal allograft tolerance without causing myelosuppressive complications.
| Group | n | TBI | TI | Bcl-2i | chimerism* | Graft Survival (days) |
| 1 | 4 | 1.5 | 7 | + | 4/4 | >313, >187, >110, >75 |
| 2 | 2 | 0 | 7 | + | 0/2 | 142, 75 |
| 3 | 3 | 1.5 | 0 | + | 3/3 | 199, 163, 97 |
| 4 | 8 | 3.0 | 7 | - | 7/8 | >1710, >1167, 837, 755, 401, 373, 206, 58 |
| 5 | 2 | 1.5 | 7 | - | 0/2 | 29, 56 |