“First-In-Human” Clinical Trial Employing Adoptive Transfer of Autologous Thymus-Derived Treg Cells (thyTreg) to Prevent Graft Rejection in Heart-Transplanted Children
Esther Bernaldo de Quirós1, Manuela Camino2, Nuria Gil2, Esther Panadero2, Constancio Medrano2, Juan Miguel Gil-Jaurena3, Marjorie Pion1, Giovanna Lombardi4, Megan K Levings5,7, Lori J West5,7, Rafael Correa-Rocha1.
1Laboratory of Immune-regulation, 'Gregorio Marañón' Health Research Institute (IISGM) , Madrid, Spain; 2Pediatric Cardiology Unit, 'Gregorio Marañón' Health Research Institute (IISGM) , Madrid, Spain; 3Pediatric Cardiac Surgery Unit, 'Gregorio Marañón' Health Research Institute (IISGM) , Madrid, Spain; 4MRC Centre for Transplantation, King's College, London, United Kingdom; 5Childhood Diseases Research Theme, BC Children's Hospital, Vancouver, BC, Canada; 6Alberta Transplant Institute, University of Alberta, Edmonton, AB, Canada; 7Canadian National Transplant Research Program, (CNTRP), Alberta, AB, Canada
Introduction: Immune allograft rejection remains the main obstacle to reach successful transplants. Transfer of regulatory T cells (Treg) has acquired growing interest in attempts to prevent rejection. However, the limited number and the differentiated phenotype of Tregs isolated from blood constitute important drawbacks for the effectiveness of this strategy. In collaboration with several teams, we have explored the use of the thymic tissue, which is routinely discarded during pediatric cardiac surgery, as an alternative source of Tregs to be used as cellular immunotherapy in heart-transplanted children.
Material and methods: We developed a novel GMP-compatible protocol to obtain massive amounts of thymus-derived Tregs (thyTreg) from thymuses discarded from infants (<3 years old). Several quality tests were performed on the final thyTreg cell product. A “first-in-human” clinical trial (phase 1/2a) will be initiated in January 2018 to test the safety, feasibility and effectiveness of the adoptive transfer of autologous thyTregs in heart-transplanted children.
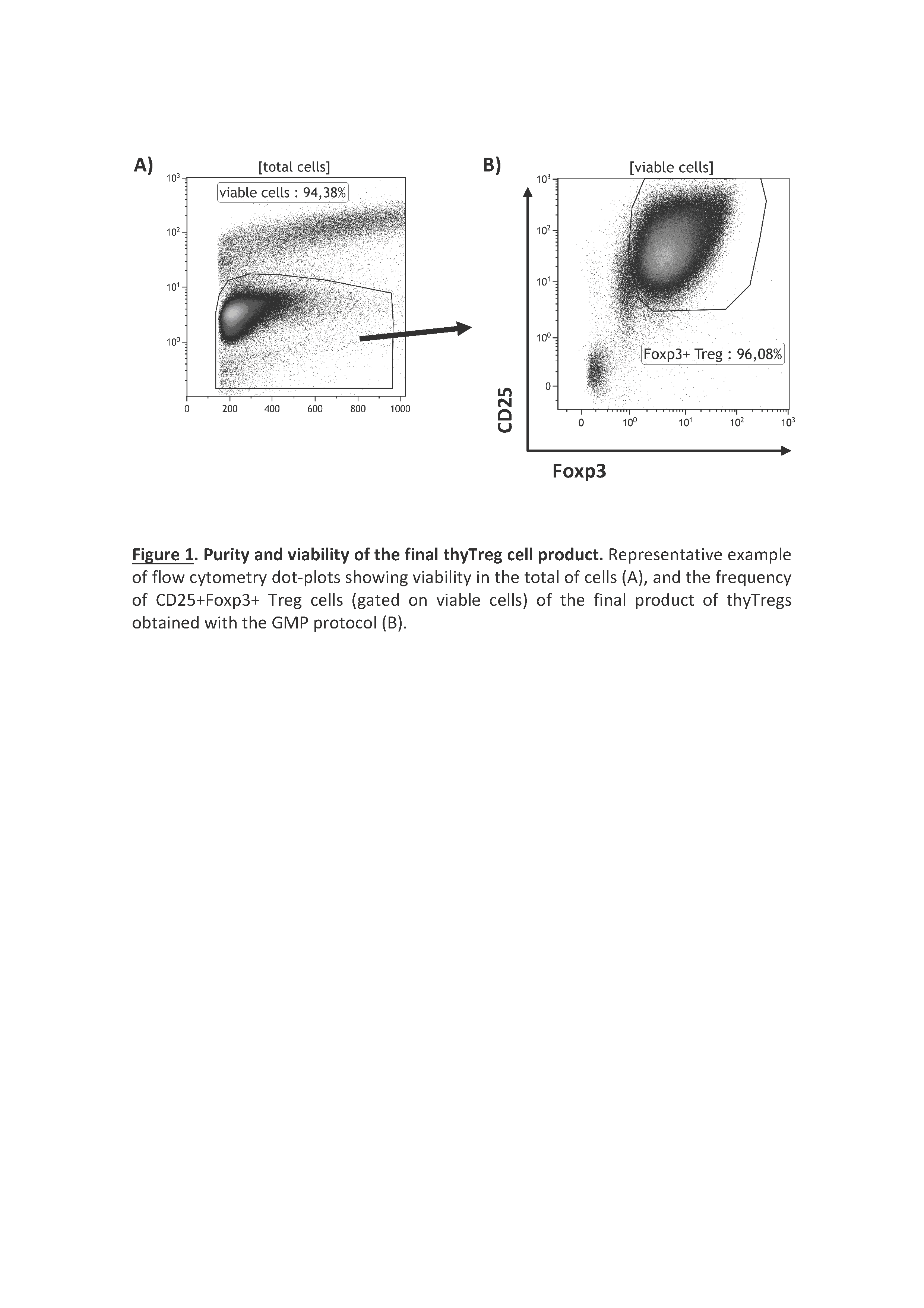
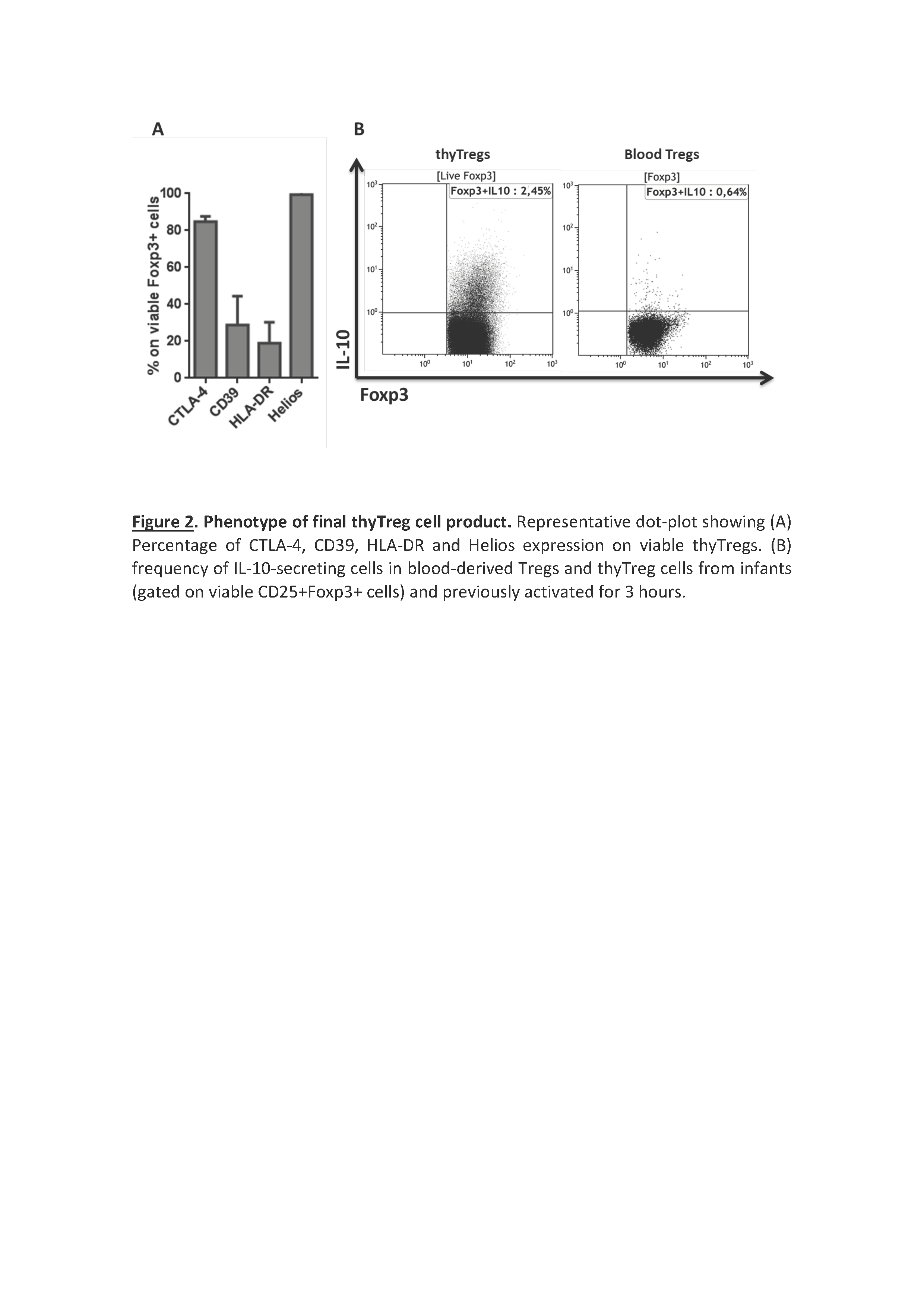
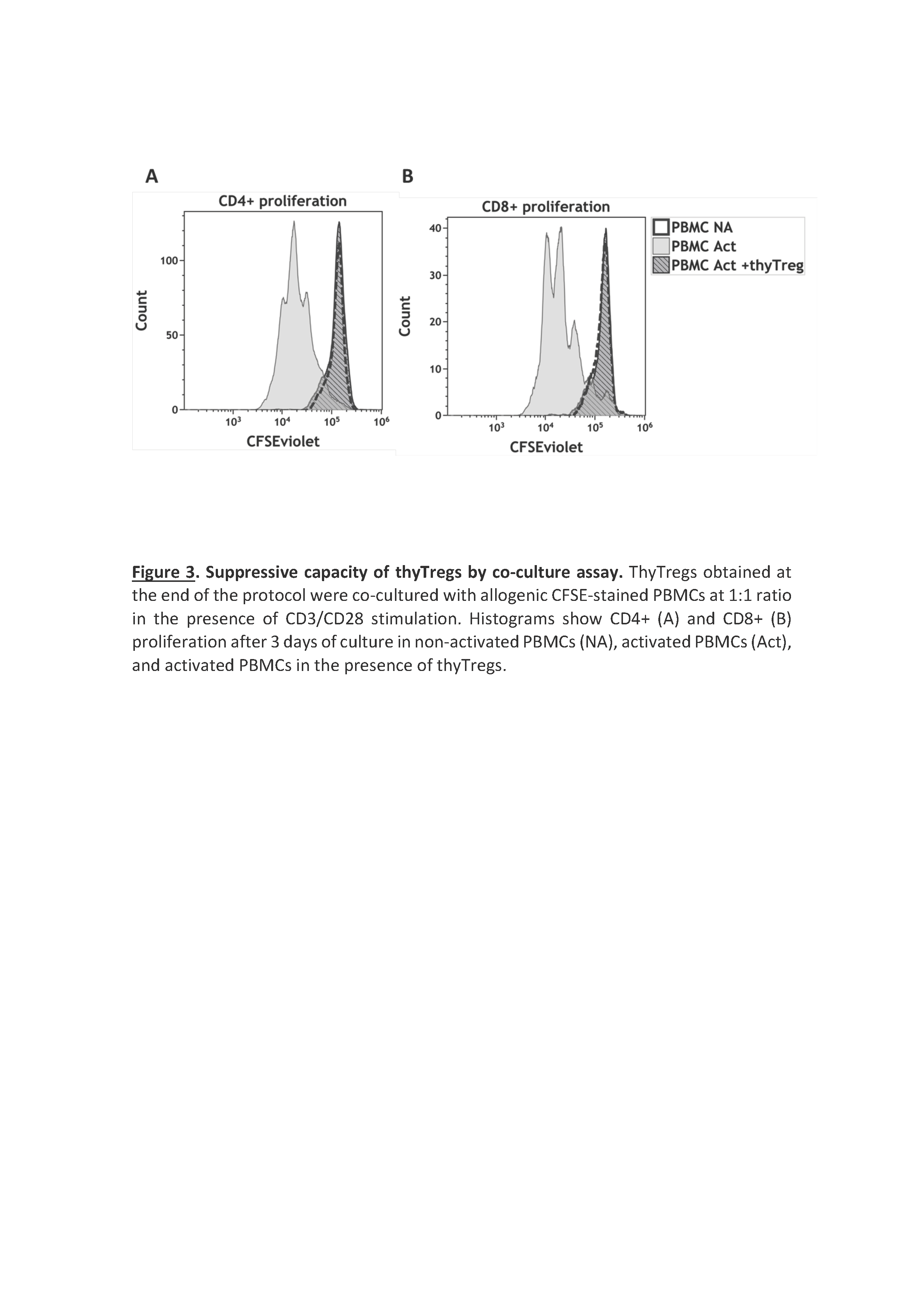
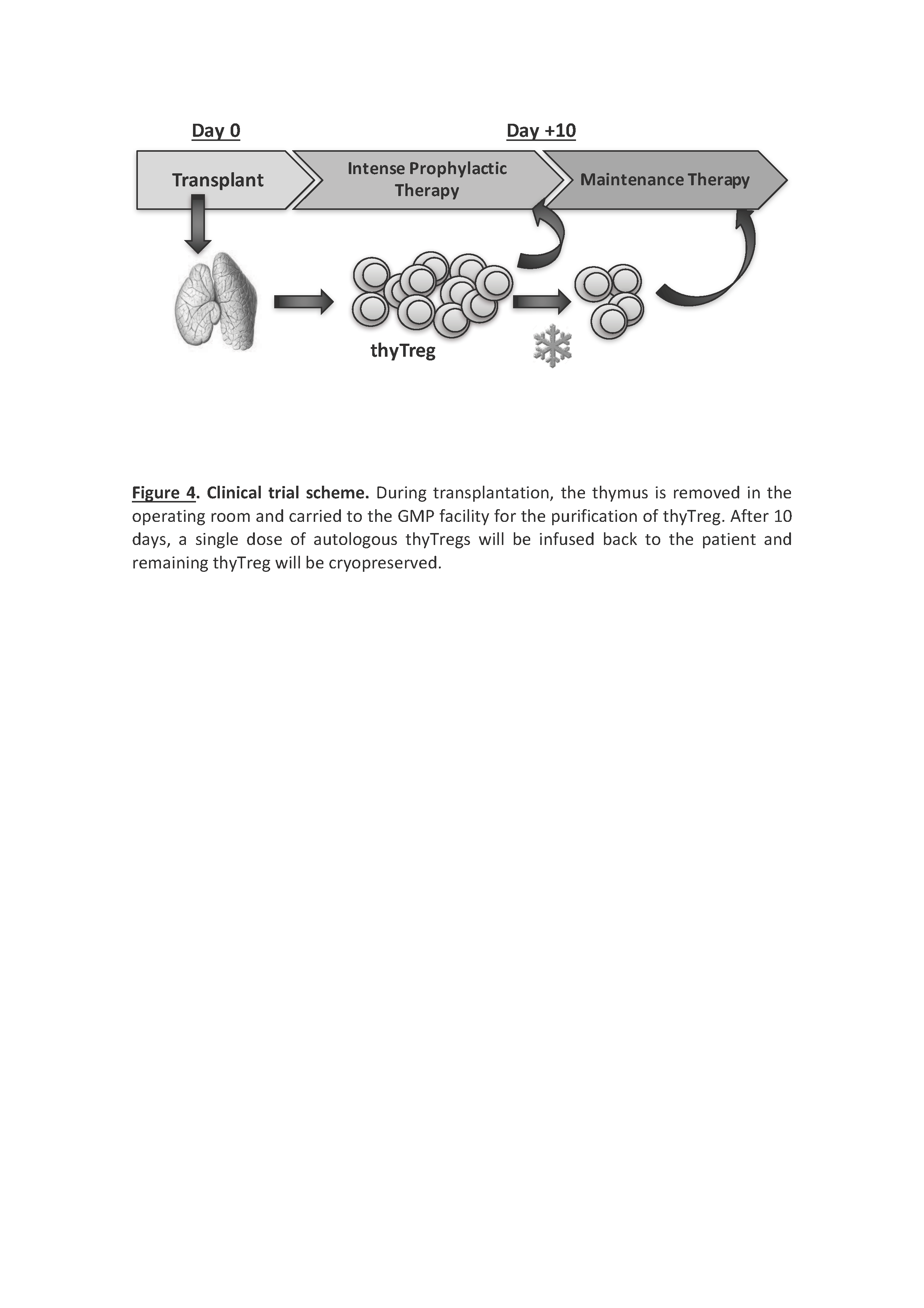
Results/Discussion: ThyTreg purified from thymuses were activated and cultured ex-vivo for 7-10 days with our protocol, and the final product showed a very high purity, with >95% of CD25+Foxp3+ cells, and a viability >90% (Fig 1). Importantly, the number of thyTreg cells obtained from one single thymus reached values of more than 13x109 (billions) cells. Considering the Treg doses employed in previous clinical trials (1-10 x106 Treg/kg), in the case of transplanted infants, this amount will be enough to prepare more than 1000 doses of thyTreg treatment. The final product of thyTreg showed great expression of CTLA-4, CD39, HLA-DR and Helios. In comparison to blood-derived Tregs, the frequency of IL-10-secreting cells was markedly higher in thyTregs (Fig 2). Besides, the final thyTreg product showed a very high suppressive capacity, decreasing the proliferation of CD4+ and CD8+ T cells by more than 80% (Fig 3). The thyTreg product will be employed as immunotherapy to prevent rejection in a clinical trial. Infants younger than 3 years old included in the waiting list for a heart transplant will be enrolled. A single dose of autologous thyTreg cells purified from the thymus discarded in the surgery will be infused back to the children at day +10 post-transplant, when doses of immunosuppressants are reduced (Fig 4). The rest of the thyTreg doses will be cryopreserved in a Biobank for potential reinfusions in the future to the patient.
Conclusion: Massive quantities of highly suppressive and pure Thy-Tregs obtained with our novel GMP-compatible protocol are suitable to be employed as cellular immunotherapy to prevent rejection in heart-transplanted children. At the beginning of 2018, we will initiate the first clinical trial to test the safety of the procedure, the feasibility and the effect of the thyTreg therapy in the context of solid organ transplantation.
Grant from Instituto de Salud Carlos III (ISCIII) co-financed by FEDER funds (PI15/00011). Grant from Instituto de Salud Carlos III (ISCIII) co-financed by FEDER funds (ICI14/00282).
