mTORC2 Deficiency in CD11c+ Dendritic Cells Promotes Acute Kidney Injury
Helong Dai1,2, Alicia Watson PhD1, Daniel Fantus MD1, Longkai Peng MD, PhD2, Angus Thomson PhD1,4, Natasha Rogers MD, PhD1,3.
1Department of Surgery, Thomas E. Starzl Transplantation Institute, University of Pittsburgh School of Medicine, Pittsburgh , PA, United States; 2Department of Urological Organ Transplantation, The Second Xiangya Hospital of Central South University, Changsha, P.R. China; 3Center for Transplant and Renal Research, Westmead Institute for Medical Research, Westmead, Australia; 4Department of Immunology, University of Pittsburgh School of Medicine, Pittsburgh , PA, United States
Introduction: Dendritic cells (DC) are critical initiators of innate immunity in the kidney and orchestrate inflammation subsequent to ischemia-reperfusion injury (IRI). The role of mammalian/mechanistic target of rapamycin (mTOR) in the pathophysiology of renal IRI has been poorly characterized; furthermore, the influence of DC-based alterations in mTOR signaling has not been investigated.
Methods: We initially evaluated activation of the mTORC1/2 pathway in sham-operated and post kidney IRI C57BL/6 mice. Ex vivo isolated bone marrow-derived mTORC2 deficient (Rictor-/-) or wild-type (WT) DC underwent hypoxia-reoxygenation and then were analysed by flow cytometry. Adoptive transfer of WT or Rictor-/- DC to C57BL/6 mice followed by unilateral renal IRI was used to assess migratory capacity. Age- and gender-matched DC-specific Rictor-/- mice or littermate controls underwent bilateral renal IRI followed by assessment of renal function, histopathology, bio-molecular and cell infiltration analysis.
Results: Protein expression of phosphorylated S6K that is a downstream of mTORC1 was upregulated, but conversely, phosphorylated Akt S473 that is a downstream of mTORC2 was decreased in whole kidney tissue in response to IRI. Rictor-/- DC express more CD80/CD86 but less programed death ligand-1 (PDL1) which can be enhanced by hypoxia-reoxygenation, and demonstrate increased migration to the injured kidney. Following IRI, Rictor-/- DC mice develop higher serum creatinine, 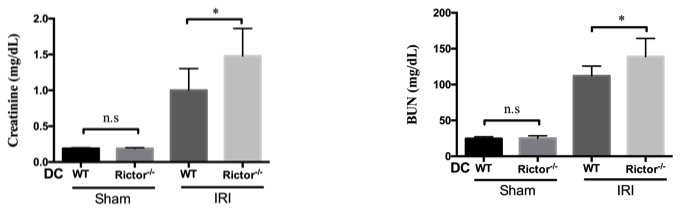 more severe histologic damage
more severe histologic damage 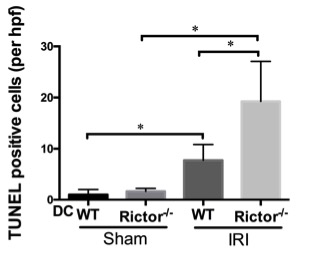 and greater pro-inflammatory mRNA transcript profiles of IL-1β, IL-6 and TNF-α compared to littermate controls.
and greater pro-inflammatory mRNA transcript profiles of IL-1β, IL-6 and TNF-α compared to littermate controls. 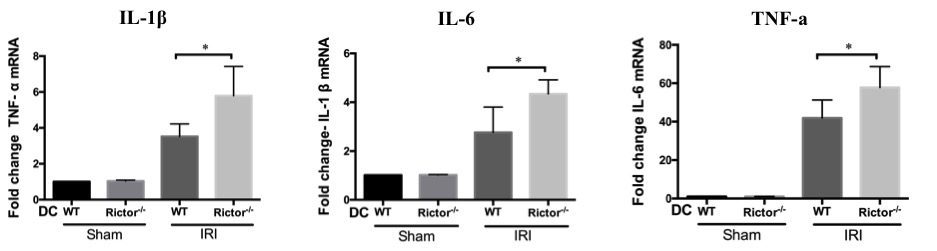 After IRI challenge, Rictor-/- DC show a trend towards increased TNF-α production, but significantly reduced IL-10 secretion compared to WT DC. A greater influx of both neutrophils and T cells was also seen in Rictor-/- DC mice, in addition to CD11c+MHCII+CD11bhiF4/80+ renal DC, which expressed more CD86 but less PDL1.
After IRI challenge, Rictor-/- DC show a trend towards increased TNF-α production, but significantly reduced IL-10 secretion compared to WT DC. A greater influx of both neutrophils and T cells was also seen in Rictor-/- DC mice, in addition to CD11c+MHCII+CD11bhiF4/80+ renal DC, which expressed more CD86 but less PDL1.
Conclusion: These novel data suggest that mTORC2 signaling in renal DC negatively regulates acute kidney injury. Thus, DC-targeted elimination of Rictor enhances inflammatory and migratory responses to the injured kidney, highlighting the regulatory roles of both DC and Rictor in the pathophysiology of renal IRI.
HD was in receipt of China Scholarship Council funding (201506370079). DF was in receipt of an American Society of Nephrology Ben Lipps Postdoctoral Research Fellowship. We appreciate Drs. Heth Turnquist, Bala Ramaswami, Mark A. Ross and Alan F. Zahorchak for valuable advice and technical support..
