A Prospective Multi-Center Observational Trial to Evaluate a CMV-specific ELIspot Assay in Solid Organ Transplant (SOT) Recipients: The PROTECT Study
Deepali Kumar1, Peter Chin-Hong2, Liise Kayler3, David Wojciechowski4, Ajit P Limaye5, Osama Gaber6, Simon Ball7, Aneesh Mehta8, Ted ` Blanchard9, Camille Kotton10.
1Transplant Infectious Diseases & Multi-Organ Transplant Program, University Health Network, Toronto, ON, Canada; 2Infectious Disease, UCSF, San Francisco, CA, United States; 3Kidney and Pancreas Transplant Program, Erie County Medical Center, Buffalo, NY, United States; 4Nephrology, Massachusetts General Hospital, Boston, MA, United States; 5Infectious Disease , University of Washington , Seattle, WA, United States; 6Transplant Surgery, Houston Methodist , Houston, TX, United States; 7Renal Medicine, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom; 8Infectious Disease, Emory University, Atlanta, GA, United States; 9Oxford Immunotec, Marlborough, MA, United States; 10Infectious Disease, Massachusetts General Hospital, Boston, MA, United States
Introduction: CMV replication in transplant recipients is primarily controlled by the T-cell response. We evaluated the role of a novel CMV-specific ELISPOT assay to predict protection against CMV infection in SOT recipients.
Materials and Methods: This was a multi-center (43 sites), prospective, observational study of kidney transplant recipients at risk for CMV. Subjects were enrolled either pre-transplant or during initial anti-viral prophylaxis. Clinical management was performed according to local institutional protocols. Cell-mediated immunity (CMI) was determined using an ELISPOT assay that evaluated responses to CMV-specific antigens IE-1 and pp65, from the capture of IFN-γ enumerated as spot counts (T-SPOT.CMV, Oxford Immunotec). CMV CMI and quantitative PCR (Roche Cobas) were assessed at 1, 2, 3, 4 and 6 months following completion of anti-viral prophylaxis. CMV events necessitated a change in anti-viral therapy or immunotherapy to be eligible for analysis. The endpoint was the first occurrence of CMV disease or CMV infection after the completion of prophylaxis. T-SPOT values taken at the completion of prophylaxis were analyzed to predict protection against CMV up to 1 year post-transplant.
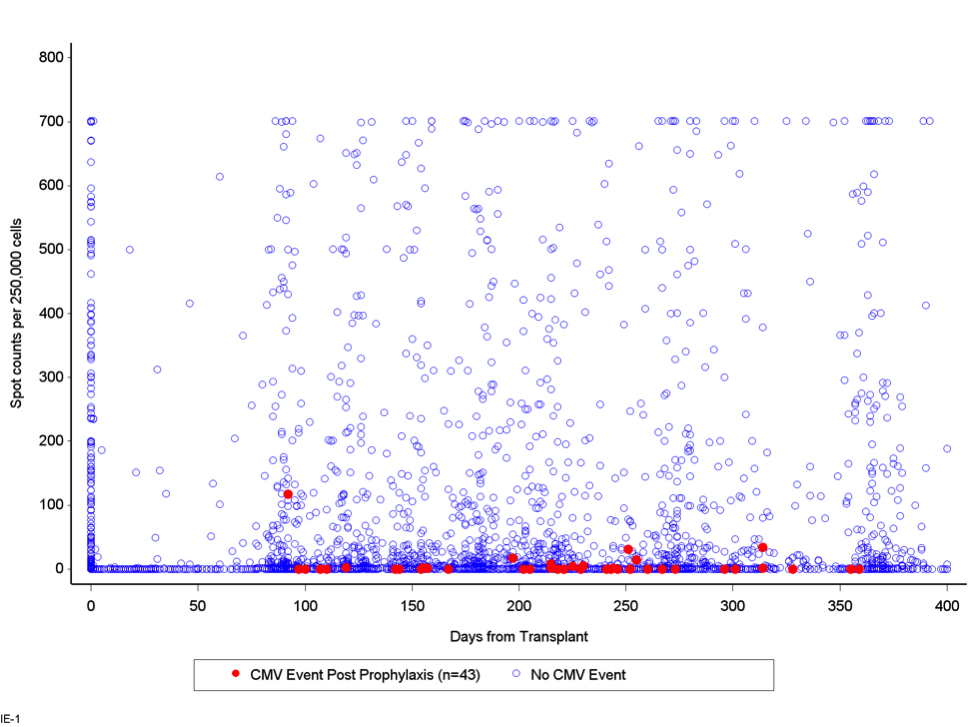
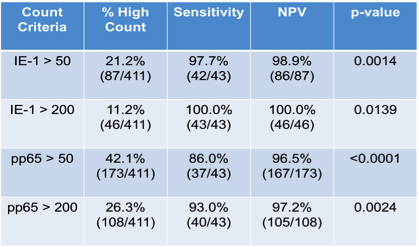
Results: Of the 597 subjects enrolled, 411 were included in the analysis based on having valid TSPOT counts within ± 30 days of the completion of prophylaxis and a non-missing date of completion of prophylaxis. Of the 411 subjects, 203 were R+ (49%), 178 were D+/R- (43%), 22 were D-/R- (5%) and 8 subjects had unknown CMV serostatus. The majority of patients were white (67%), male (62%), and median age was 52. Most patients received 3 months of antiviral prophylaxis (55%) vs 6 months of prophylaxis (44%). 3 subjects did not receive any antiviral prophylaxis (1%). Of the 411 eligible subjects, 87 had an IE-1 response of > 50 spots at the completion of prophylaxis. In this group, 1 of 87 developed CMV infection following the completion of prophylaxis, resulting in a negative predictive value (NPV) against the occurrence of a CMV event of 98.9% (p=0.0014) (see Figure 1). Using the same cut-off for pp65, the NPV against the occurrence of a CMV event was 96.5% (p=<0.0001) (see Figure 2).
Conclusion: To date, this is the largest prospective study on the use of IFN-γ release assays to predict the risk of CMV infection in organ transplant recipients. We show that T-SPOT.CMV IE-1 and pp65 spot counts > 50 at the completion of prophylaxis are a significant predictor of protection against CMV infection/disease in SOT recipients.