Living Donor Liver Transplantation for Alcoholic Liver Disease: The North American Experience
Hillary Braun1, Jennifer L Dodge MPH1, Josh D Grab MS1, Shareef M Syed MD1, Garrett R Roll MD1, Chris E Freise MD1, John P Roberts MD1, Nancy L Ascher MD, PhD1.
1Surgery, University of California, San Francisco, San Francisco, CA, United States
Introduction: Alcoholic liver disease (ALD) is now a well-recognized indication for liver transplantation, however the literature on living donor liver transplantation (LDLT) for ALD is sparse and primarily from single Asian transplant centers. Here, we report LDLT outcomes for ALD from the Adult-to-Adult Living Donor Liver Transplantation Study (A2ALL), comprising data from nine transplant centers in North America.
Materials and Methods: Demographics and outcomes of donors and recipients in the A2ALL study who underwent LDLT between April 1998 and January 2014 were reviewed. We compared recipients with ALD and non-ALD diagnoses using Wilcoxon rank sum and chi-squared tests; graft and patient survival were estimated using Kaplan-Meier methods and compared by the log-rank test.
Results: 1065 donor/recipient pairs were included. 168 (15.8%) recipients underwent LDLT for ALD; the majority were male (70.8%) and Caucasian (92.7%), with a median (IQR) age of 53.3 (47.5-59.0) years, BMI of 26.2 (23.2-29.5), and MELD of 15 (13-19). 94.6% of recipients received a right lobe graft and 70.8% of recipients were biologically related to their donor. Compared with recipients who underwent LDLT for other etiologies of liver disease, those with ALD had a greater proportion of male recipients (70.8% vs. 55.9%, p<0.001) and concomitant HCV diagnosis (44.6% vs. 36.6%, p=0.048). ALD recipients were significantly older (53vs.52years, p=0.02) with a greater weight (79.2vs.75.0 kg, p=0.04). There was no difference in median BMI or MELD. Post-operatively, there was no significant difference in median number of complications between ALD vs. non-ALD (3 vs. 3, p=0.75), nor was there a difference in highest Clavien grade (2 vs. 2, p=0.23), rejection (8.4% vs. 11.1%, 0.39), hernia (25.3% vs. 19.7%, p=0.12), HAT (5.8% vs. 7.5%, p=0.48), bile leak (28.6% vs. 27.8%, p=0.85), or biliary stricture (32.5% vs. 33.3%, p=0.84). Patient survival was similar for ALD and non-ALD recipients at one (94% vs. 91%), five (83% vs. 79%), and ten (61% vs. 66%) years post-transplant (p=0.32) (Figure 1). No significant differences were detected in graft survival between ALD and non-ALD recipients at one (88% vs. 84%), five (76% vs. 74%), or ten (55% vs. 61%) years post-transplant (p=0.29) (Figure 2).
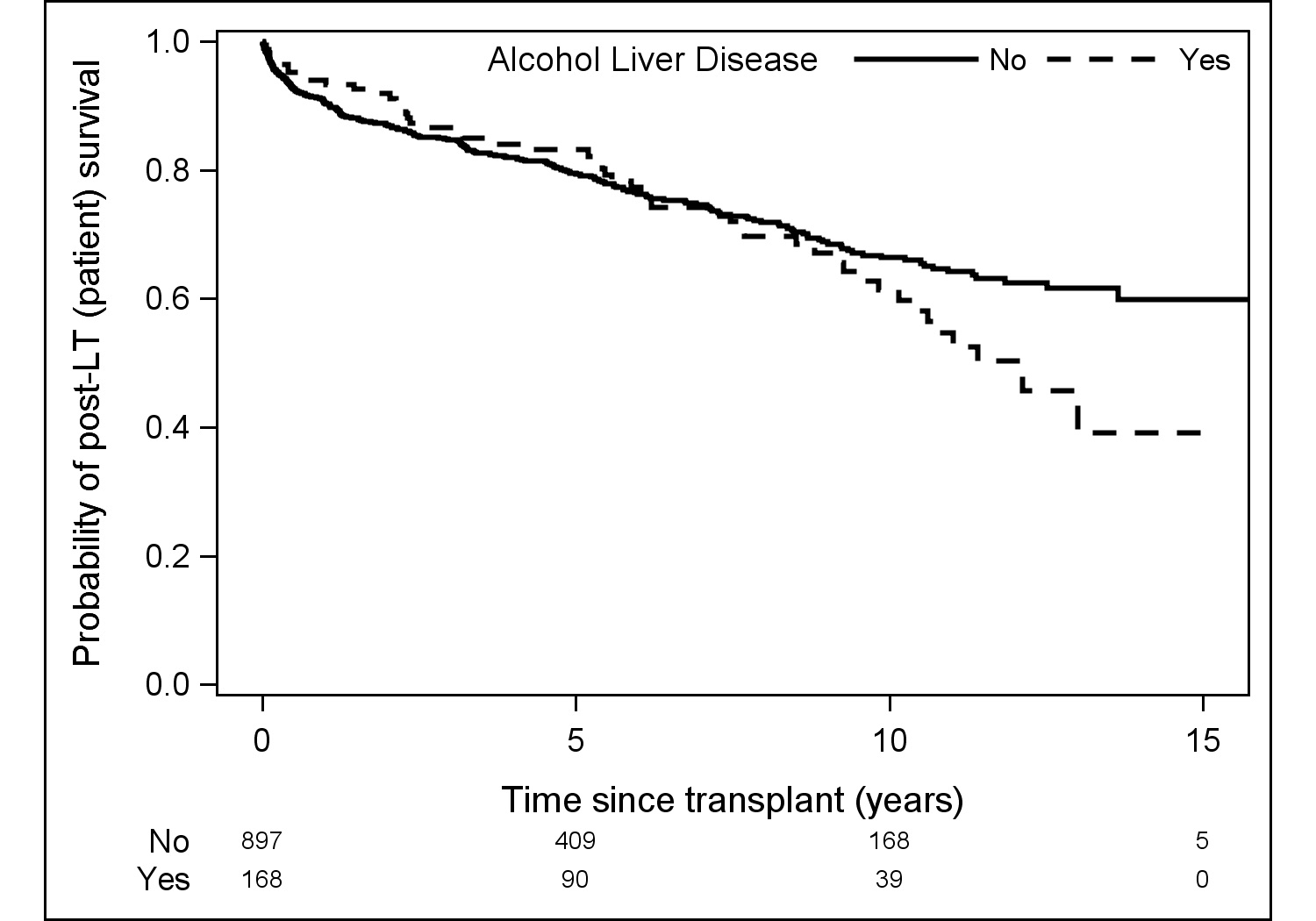
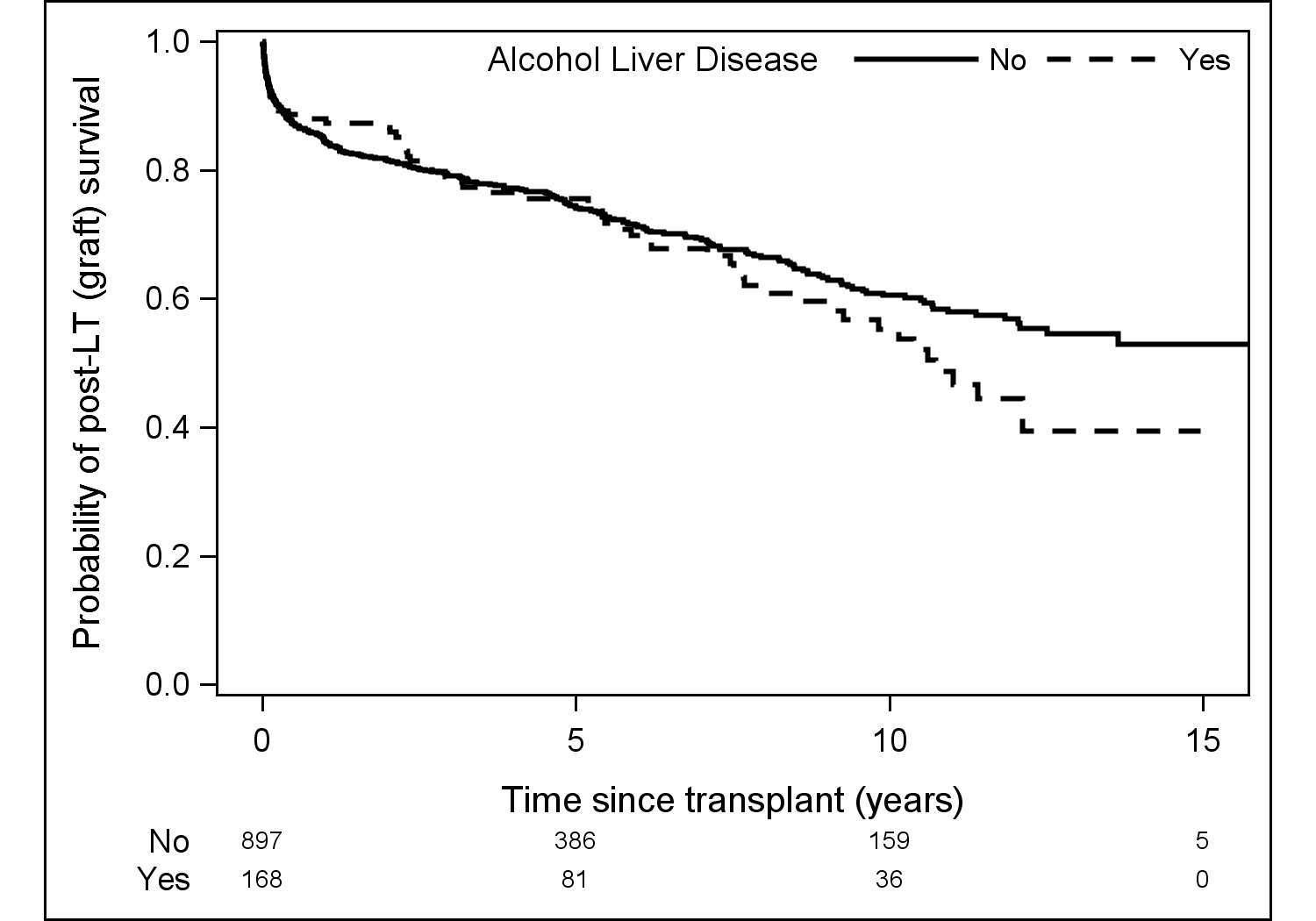
Discussion: Though there were early concerns regarding recipient outcome and potential recidivism, ALD is now a well-recognized indication for liver transplantation. While we do not have data regarding pre-transplant abstinence and post-transplant recidivism in the A2ALL cohort, we found no significant difference in major complications, patient survival, or graft survival among patients transplanted for ALD and those transplanted for other etiologies of liver disease. These findings suggest LDLT is a safe and successful option for patients who require liver transplantation for ALD.
Conclusion: As demonstrated in this large North American cohort, excellent outcomes can be achieved using LDLT for ALD.
