Ibrahim Adam, Canada has been granted the TTS-CST International Transplantation Science Mentee-Mentor Award

Antibody Response to Non-Self Blood Group A-Antigen depends on CD4 T Cells, Forigen Protein and CD22 Interaction
Ibrahim Adam1,4,5, Bruce Motyka2,4, Jean Pearcey2,4, Kesheng Tao2,4, Peter Cowan6, Lori West1,2,3,4,5.
1Medical Microbiology and Immunology, University of Alberta, Edmonton, AB, Canada; 2Pediatrics, University of Alberta, Edmonton, AB, Canada; 3Surgery, University of Alberta, Edmonton, AB, Canada; 4Alberta Transplant Institute, University of Alberta, Edmonton, AB, Canada; 5Canadian National Transplant Research Program, University of Alberta, Edmonton, AB, Canada; 6St. Vincent Hospital, University of Melbourne, Melbourne, Australia
Background: ABO-incompatible heart transplantation (ABOi-HTx) is safe during infancy and allows increased donor access. B-cell tolerance develops to donor A/B-antigen(s) (Ag) after ABOi-HTx by mechanisms remaining unclear. We developed transgenic mice (A-Tg) constitutively expressing human A-Ag on vascular endothelium and erythrocytes (RBC) to study anti-A antibody responses. CD22 participates in B-cell tolerance and we found that B cells express high-levels of CD22 in human B cells, decreasing with age. Here we used a mouse model to study the anti-A response in the context of MHC syngeneic, allogeneic and xenogeneic stimulation, and the impact of CD22 expression.
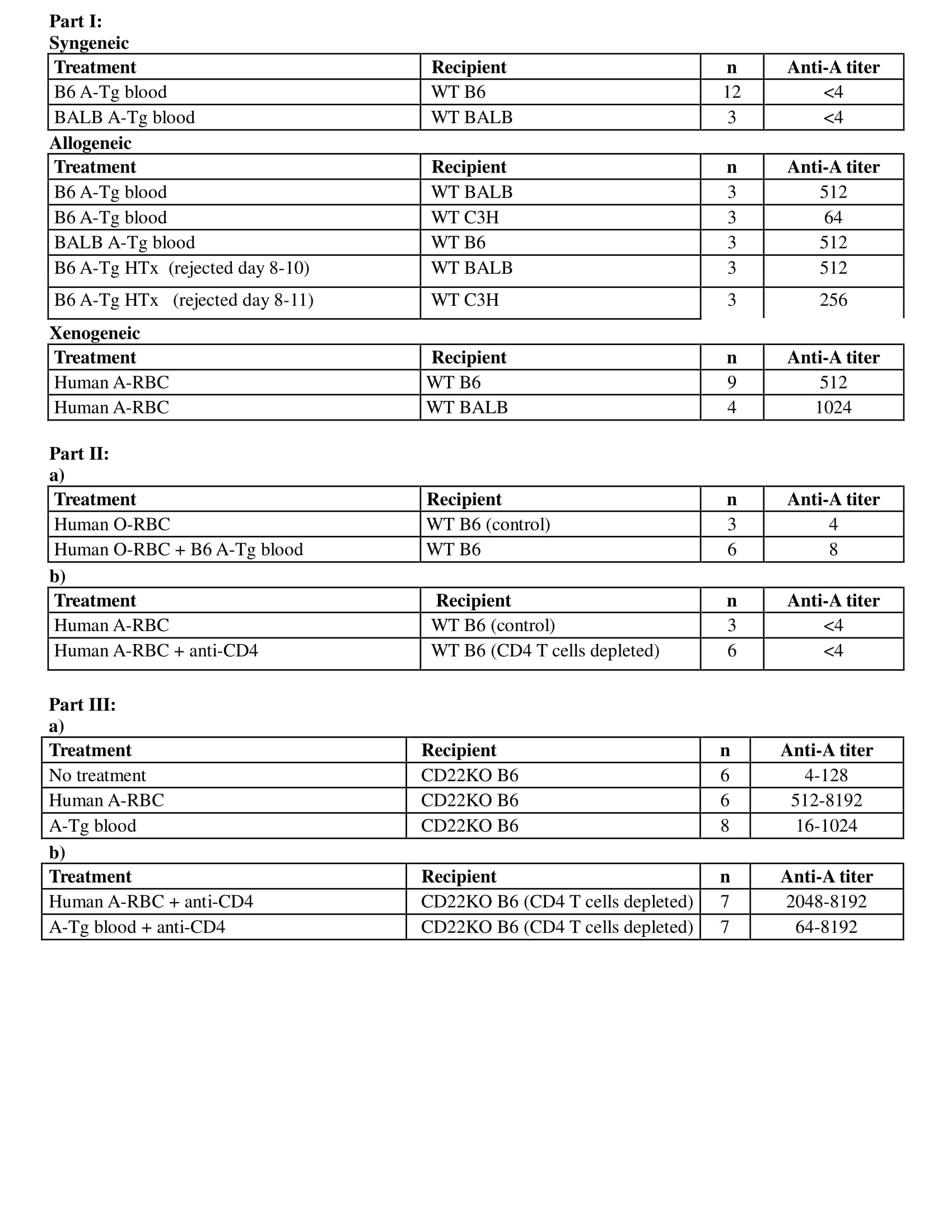
Methods: Part I: Adult wild-type (WT) C57BL/6 (B6/H-2b), BALB/c (BALB/H-2d), C3H/He (C3H/H-2k), or CD22-deficient B6 (CD22KO) mice received intraperitoneal injections of B6 or BALB A-Tg blood cells or human-RBC membranes (100ul/10%v/v) from blood group-A (hu-A) or O (hu-O); or A-incompatible heart allografts. Serum anti-A Ab was measured by hemagglutination and ELISA (IgG and IgM); graft survival was assessed by palpation. Part II: a) To assess requirement of foreign protein to stimulate anti-A, hu-O RBC/syngeneic A-Tg cells or allogeneic A-Tg blood were co-injected in WT mice; b) to assess T cell dependence of anti-A response, CD4+ T cells were depleted from WT B6 mice before hu-A RBC injection. Part III: To assess the role of CD22, A-Tg or hu-A-RBC, were injected into CD22KO mice with or without CD4+ T-cell depletion.
Results: Part I: Exposure to allogeneic A-Tg blood cells/heart graft or xenogeneic hu-RBC induced anti-A production (Table), whereas syngeneic A-Tg blood cells did not. Part II: a) mixture of syngeneic A-Tg/hu-O RBC did not induce anti-A; b) after CD4+ T-cell depletion, hu A-RBC failed to elicit anti-A. Part III: Hu A-RBC induced a very high anti-A in CD22KO mice compared to WT B6. In contrast to WT B6 mice, anti-A Ab was elicited in CD22KO mice following injection with A-Tg blood cells or hu A-RBC with CD4+ T cell depletion.
Conclusions: Our results show that in WT mice, anti-A antibody production depends not only on exposure to A-antigen but also co-engagement with foreign protein and a requirement for CD4+ cells; consistent with a T-dependent anti-A response. Conversely, in CD22KO mice there was no requirement for foreign protein or CD4+ cells to elicit an anti-A antibody response; consistent with a T-independent anti-A response. These findings suggest an important role for the regulatory CD22 receptor in the B cell response to ABH antigens.