DSA with TCMR Identifies High Risk Renal Transplant Recipients that can be Predicted by Proinflammatory Transitional B Cells
Dominik Chittka1, Aravind Cherukuri1, Akhil Sharma1, Rajil Mehta1, Sundaram Hariharan1, David M Rothstein1.
1Thomas E. Starzl Transplant Institute, University of Pittsburgh, Pittsburgh, PA, United States
Introduction. Post-transplant DSA is strongly associated with poor graft outcomes but has limited predictive value. In this prospective study, we aimed to risk stratify patients with early post-transplant DSA to allow timely identification of individuals at risk for poor clinical outcomes.
Methods. Patients were screened for DSA at 0, 1, 3, 6, 9 & 12 months and analyzed in relation to protocol biopsies at 3 and 12 months and any for-cause biopsies within the first year. To risk stratify DSA positive patients, we analyzed B cell subsets and cytokines of those who had PBMC available at 3 months.
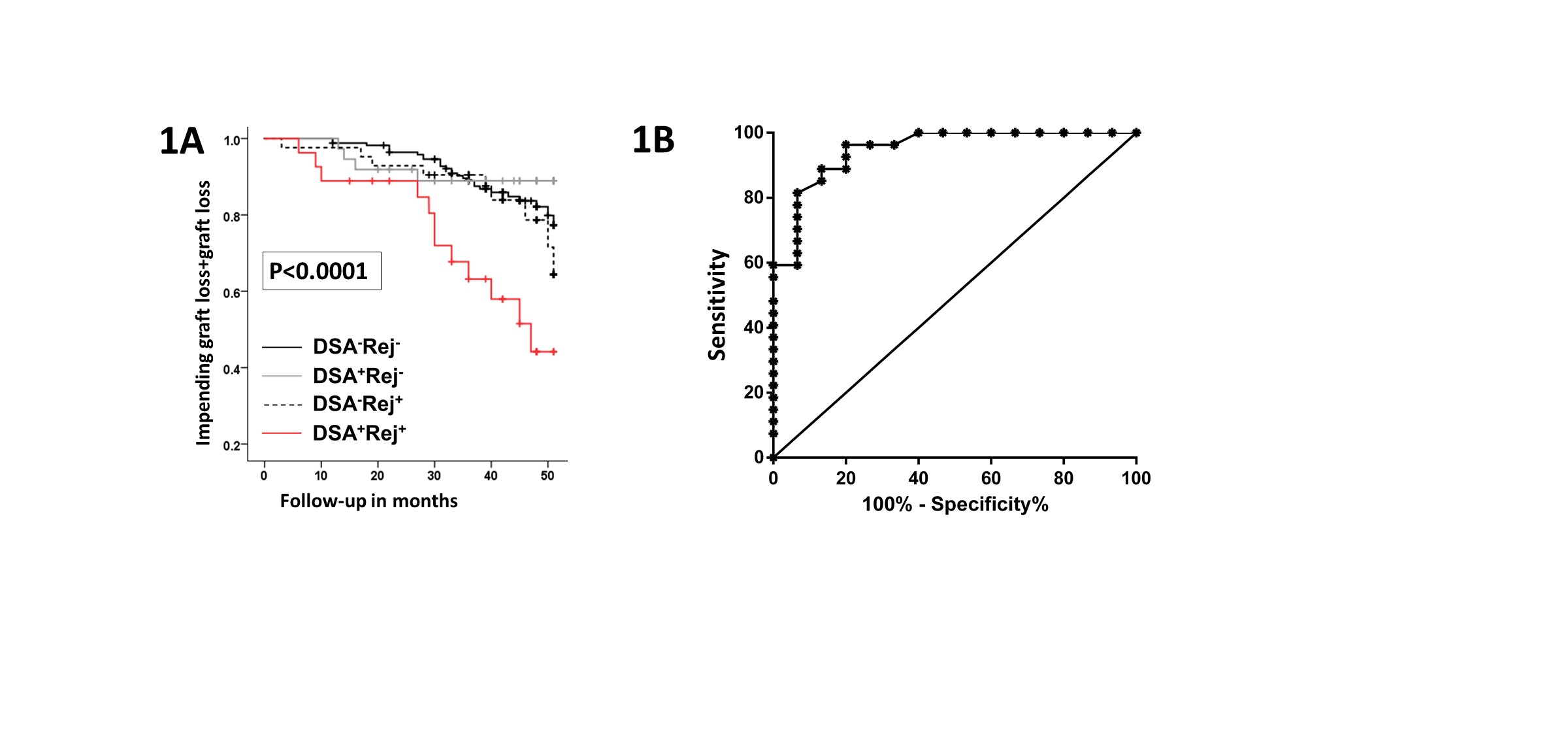
Results. 294/ 372 of patients transplanted between 01/2013 and 11/2014 with at least one biopsy in the first post-transplant year were included in the analysis. The immunosuppressive regimen was Thymoglobulin induction followed by MPA and Tacrolimus as maintenance therapy. 67/294 (22.8%) of these patients developed DSA. DSA was detected early (< 3 months) in 76% of the patients. DSA was associated with significantly increased rates of subclinical and clinical TCMR (58%) compared to patients lacking DSA (33%, %; p<0.0001). Importantly, patients with DSA plus TCMR had significantly worse chronic allograft changes (1 year protocol biopsy) and increased graft loss or impending graft loss (eGFR < 30ml/min and > 30% decline from baseline) at 4 years compared to those with DSA or TCMR alone (Fig. 1A). Thus, DSA plus TCMR identifies high-risk patients, in whom early identification would allow pre-emptive intervention.
Based on prior findings, we asked whether cytokine expression by peripheral blood B cell subsets could predict TCMR in patients with DSA. 43/67 of DSA positive patients had their B cell cytokines analyzed at 3 months by flow cytometry (after 24 h stimulation with CpG and CD40L). Of the markers analyzed, the ratio of IL-10/TNFα expression by T1 transitional B cells (T1B) was significantly lower in patients with DSA plus TCMR compared to those with DSA alone (ratio: 0.94 vs. 4.9, p<0.0001). A low T1B cytokine ratio was a strong predictor of DSA plus TCMR (ROC AUC 0.94, p<0.0001; Fig. 1B). At a cut-off value of 1.26, the T1B cytokine ratio predicted DSA plus TCMR with a positive predictive value of 81% and a negative predictive value of 94%. Reanalysis of the data after removing the 5/43 patients whose DSA was detected after TCMR again showed that the T1 B cytokine ratio strongly predicted concomitant or ensuing TCMR in patients with DSA (ROC AUC 0.94, p<0.0001).
Conclusion. Thus, patients with DSA plus TCMR represent a high-risk population for adverse graft outcomes, and this outcome can be predicted in DSA positive patients using the T1B cytokine ratio as a biomarker.
Deutsche Forschungsgesellschaft (DFG). American Society of Transplantation (AST).
