DHRS9 is a Stable Marker of Human Regulatory Macrophages
Florian Bitterer1, Giada Amodio2, Camila Macedo3, Aurelie Moreau4, Natasa Obermajer5, Fred Frändrich6, Cristina Cuturi4, Silvia Gregori2, Hans J. Schlitt1, Angus W. Thomson3, Edward K. Geissler1, James A. Hutchinson1, Paloma Riquelme1.
1Surgery, University Hospital Regensburg, Regensburg, Germany; 2Stem Cells and Gene Therapy, San Raffaele Scientific Institute, Milan, Italy; 3Surgery, Thomas E. Starzl Transplantation Institute, Pittsburgh, PA, United States; 4Center for Research in Transplantation and Immunology, Université de Nantes, Nantes, France; 5Surgical Oncology, Hillman Cancer Center, Pittsburgh, PA, United States; 6Institute for Applied Cell Therapy, University Hospital of Schleswig-Holstein, Kiel, Germany
Introduction: Human regulatory macrophages (Mreg) have emerged as a promising cell type for use as a cell-based adjunct immunosuppressive therapy in living-donor kidney transplantation. A therapeutic cell product, known as Mreg_UKR, is under investigation in a Phase-I/II trial as a means of safely minimising maintenance immunosuppression. Mregs can be distinguished from macrophages in other polarisation states by their unique mode of derivation, a constellation of surface markers (Fig.1) and suppressor function; however, until now, no single marker was available to specifically and stably identify human Mregs.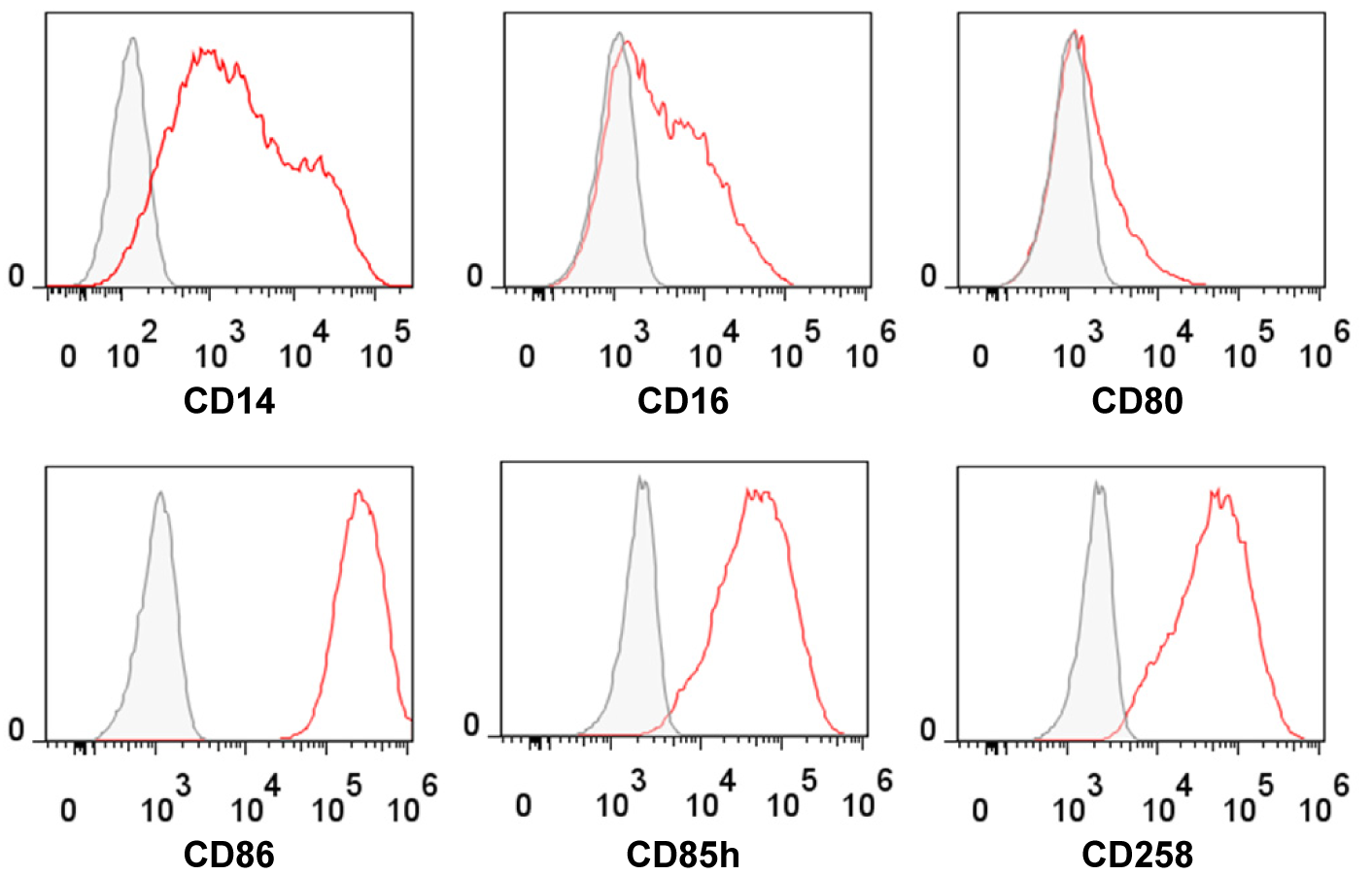
Materials and Methods: By immunoprecipitation and MALDI-MS sequencing, dehydrogenase/reductase 9 (DHRS9), a little-studied retinol dehydrogenase of the SDR family of NAD(P)(H)-dependent oxidoreductases, was identified as the cognate antigen of a mouse monoclonal antibody raised against human Mreg lysates (Fig.2). DHRS9 expression within a panel of human monocyte-derived macrophages and dendritic cells (DC) was investigated by q-PCR, immunoblotting and flow cytometry.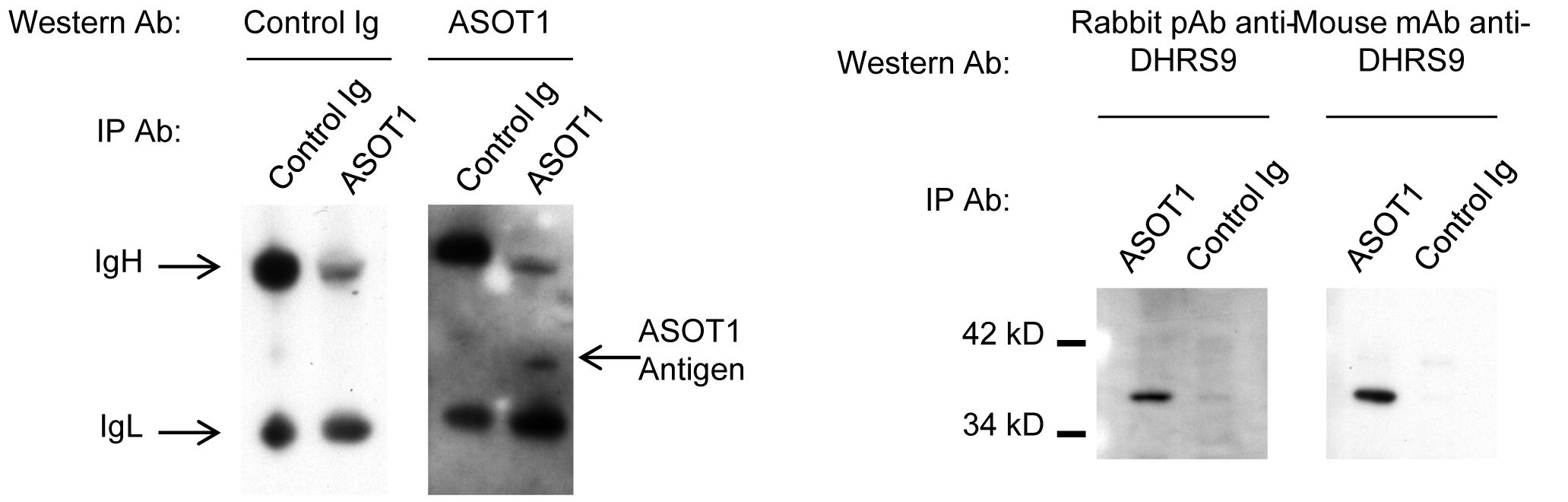
Results and Discussion: DHRS9 expression discriminated human Mregs from a diverse panel of in vitro-derived macrophages (Fig.3A&B) and human monocyte-derived tolerogenic DC, including Tol-DC, Rapa-DC, DC-10 and PGE2-induced MDSC (Fig.3C&D). 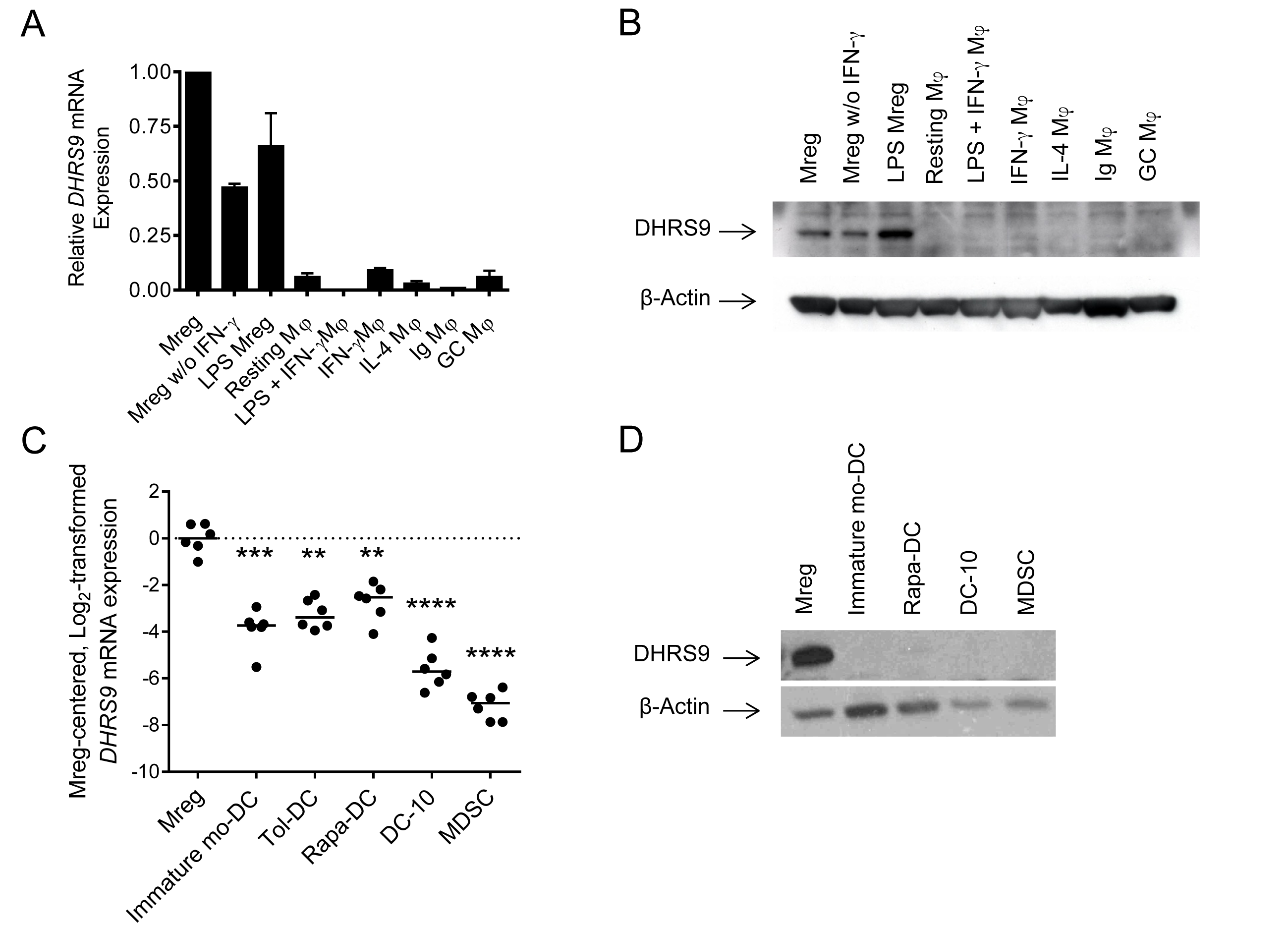 Treating Mregs with 100 ng/ml lipopolysaccharide for 24h did not extinguish DHRS9 expression. Expression of DHRS9 was acquired gradually during in vitro development of Mregs from CD14+ monocytes and was further enhanced by IFN-γ stimulation. As a member of the SDR family of retinol dehydrogenases, DHRS9 may be responsible for conversion of retinol to retinal, which is further metabolised to retinoic acid by retinal dehydrogenases, including ALDH1A1 and ALDH1A2 (Fig.4A).
Treating Mregs with 100 ng/ml lipopolysaccharide for 24h did not extinguish DHRS9 expression. Expression of DHRS9 was acquired gradually during in vitro development of Mregs from CD14+ monocytes and was further enhanced by IFN-γ stimulation. As a member of the SDR family of retinol dehydrogenases, DHRS9 may be responsible for conversion of retinol to retinal, which is further metabolised to retinoic acid by retinal dehydrogenases, including ALDH1A1 and ALDH1A2 (Fig.4A). 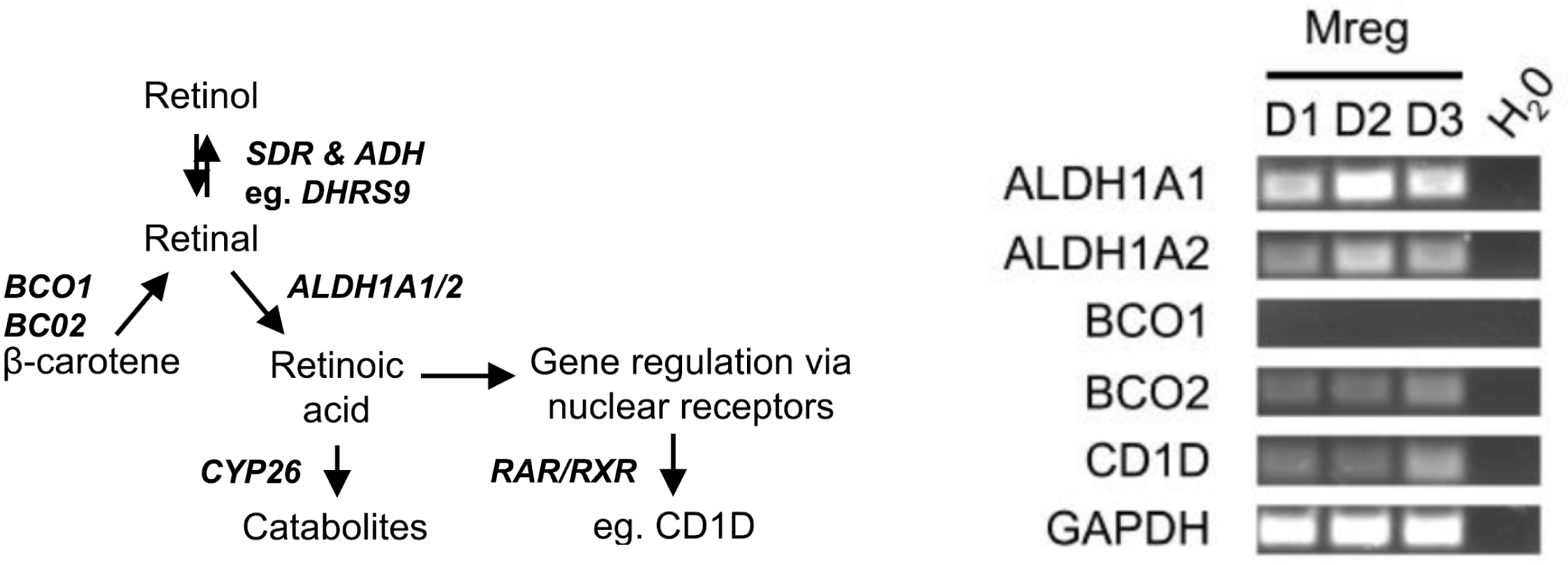 Retinol is liberated from β-carotene through the action of beta-carotene monooxygenases, such as BCO2. Accordingly, Mregs express the enzymes necessary to convert retinol and β-carotene to retinoic acid (Fig.4B). The functional significance of DHRS9 expression in Mregs has not been firmly established; however, it is well-known that certain tissue-resident macrophage populations responsible for maintaining tissue homeostasis and preventing constitutive inflammation, such as those in the gut, suppress T cell reactions and induce Tregs through production of retinoic acid. A population of DHRS9+ human splenic macrophages was identified by immunohistochemistry. Although it cannot be inferred that these naturally-occurring DHRS9+ macrophages are a physiological equivalent of in vitro-derived Mregs, existence of these cells suggests that DHRS9 expression by cultured Mregs is not an artefact.
Retinol is liberated from β-carotene through the action of beta-carotene monooxygenases, such as BCO2. Accordingly, Mregs express the enzymes necessary to convert retinol and β-carotene to retinoic acid (Fig.4B). The functional significance of DHRS9 expression in Mregs has not been firmly established; however, it is well-known that certain tissue-resident macrophage populations responsible for maintaining tissue homeostasis and preventing constitutive inflammation, such as those in the gut, suppress T cell reactions and induce Tregs through production of retinoic acid. A population of DHRS9+ human splenic macrophages was identified by immunohistochemistry. Although it cannot be inferred that these naturally-occurring DHRS9+ macrophages are a physiological equivalent of in vitro-derived Mregs, existence of these cells suggests that DHRS9 expression by cultured Mregs is not an artefact.
Conclusion: DHRS9 is a specific and stable marker of human Mregs that should be useful in future studies, especially searching for a natural counterpart of the in vitro-derived Mreg.
